Question Number 75860 by peter frank last updated on 18/Dec/19
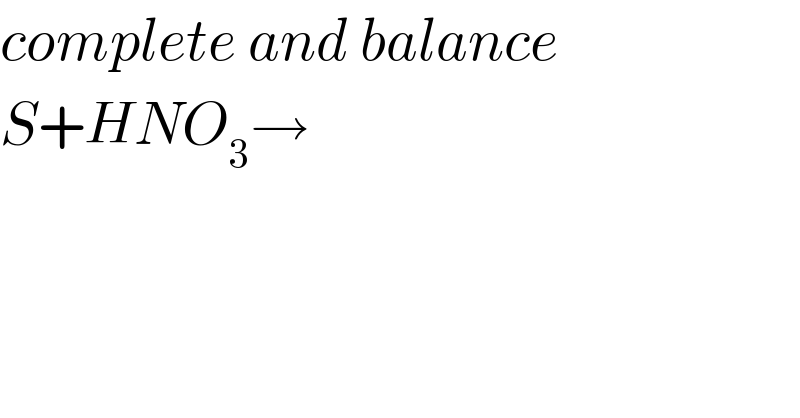
$${complete}\:{and}\:{balance}\: \\ $$$${S}+{HNO}_{\mathrm{3}} \rightarrow \\ $$$$ \\ $$
Answered by Crabby89p13 last updated on 19/Dec/19
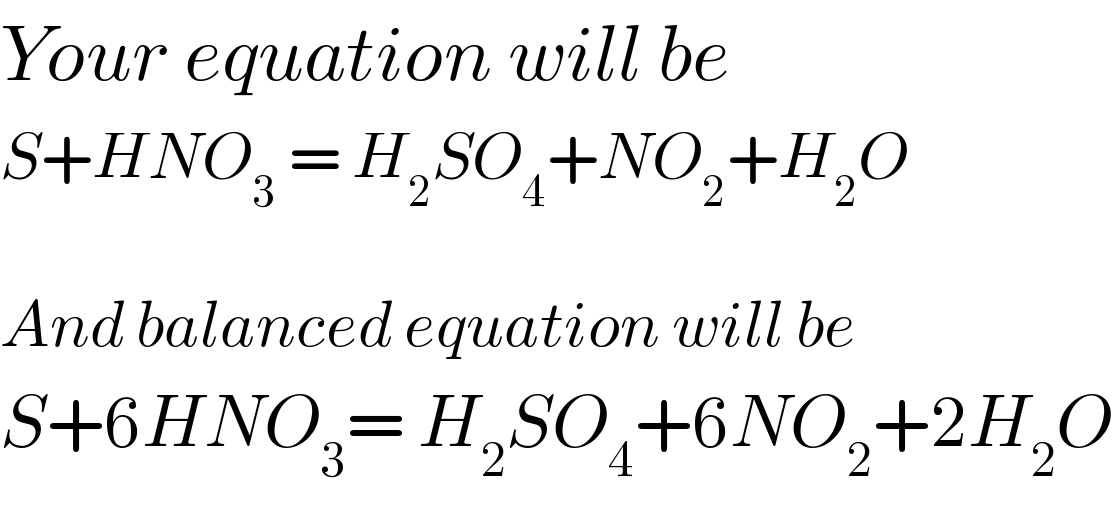
$${Your}\:{equation}\:{will}\:{be} \\ $$$${S}+{HNO}_{\mathrm{3}} \:=\:{H}_{\mathrm{2}} {SO}_{\mathrm{4}} +{NO}_{\mathrm{2}} +{H}_{\mathrm{2}} {O} \\ $$$$ \\ $$$${And}\:{balanced}\:{equation}\:{will}\:{be} \\ $$$${S}+\mathrm{6}{HNO}_{\mathrm{3}} =\:{H}_{\mathrm{2}} {SO}_{\mathrm{4}} +\mathrm{6}{NO}_{\mathrm{2}} +\mathrm{2}{H}_{\mathrm{2}} {O} \\ $$
Commented by peter frank last updated on 19/Dec/19

$${thank}\:{you} \\ $$
