Question Number 10023 by Tawakalitu ayo mi last updated on 21/Jan/17

Answered by ridwan balatif last updated on 21/Jan/17
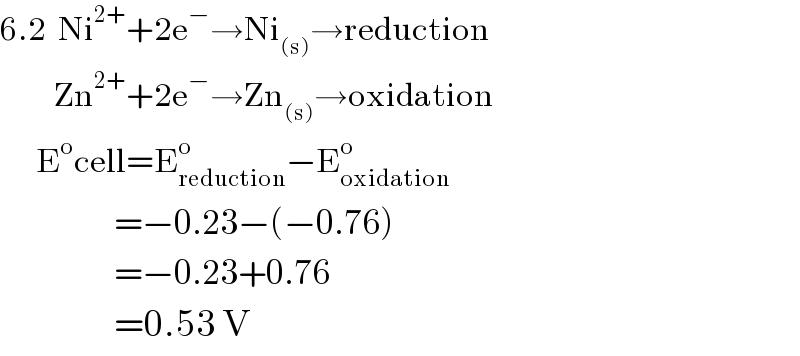
$$\mathrm{6}.\mathrm{2}\:\:\mathrm{Ni}^{\mathrm{2}+} +\mathrm{2e}^{−} \rightarrow\mathrm{Ni}_{\left(\mathrm{s}\right)} \rightarrow\mathrm{reduction} \\ $$$$\:\:\:\:\:\:\:\:\:\mathrm{Zn}^{\mathrm{2}+} +\mathrm{2e}^{−} \rightarrow\mathrm{Zn}_{\left(\mathrm{s}\right)} \rightarrow\mathrm{oxidation} \\ $$$$\:\:\:\:\:\:\mathrm{E}^{\mathrm{o}} \mathrm{cell}=\mathrm{E}_{\mathrm{reduction}} ^{\mathrm{o}} −\mathrm{E}_{\mathrm{oxidation}} ^{\mathrm{o}} \\ $$$$\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:=−\mathrm{0}.\mathrm{23}−\left(−\mathrm{0}.\mathrm{76}\right) \\ $$$$\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:=−\mathrm{0}.\mathrm{23}+\mathrm{0}.\mathrm{76} \\ $$$$\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:=\mathrm{0}.\mathrm{53}\:\mathrm{V} \\ $$
Commented by Tawakalitu ayo mi last updated on 21/Jan/17

$$\mathrm{wow},\:\mathrm{God}\:\mathrm{bless}\:\mathrm{you}\:\mathrm{sir}. \\ $$
Answered by ridwan balatif last updated on 21/Jan/17

$$\mathrm{6}.\mathrm{1}\:\mathrm{reaction}\:\mathrm{will}\:\mathrm{be}\:\mathrm{spontaneous}\:\mathrm{if}\:\mathrm{E}^{\mathrm{o}} >\mathrm{0},\:\mathrm{so} \\ $$$$\:\:\:\:\:\:\:\:\mathrm{the}\:\mathrm{answer}\:\mathrm{is} \\ $$$$\left(\mathrm{a}\right).\mathrm{spontaneous} \\ $$$$\left(\mathrm{b}\right).\mathrm{spontaneous} \\ $$$$\left(\mathrm{c}\right).\mathrm{spontaneous} \\ $$$$\left(\mathrm{f}\right).\mathrm{spontaneous} \\ $$
Commented by Tawakalitu ayo mi last updated on 21/Jan/17

$$\mathrm{i}\:\mathrm{really}\:\mathrm{appreciate}\:\mathrm{sir}. \\ $$
