Question Number 139619 by I want to learn more last updated on 29/Apr/21

Answered by mr W last updated on 30/Apr/21
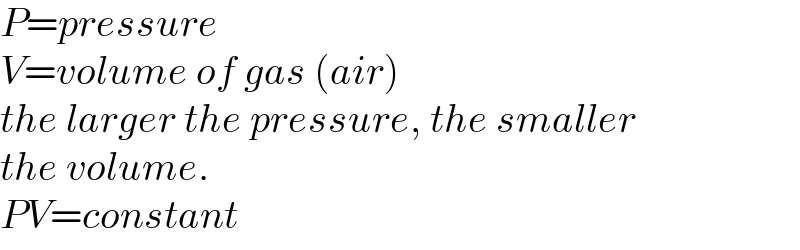
$${P}={pressure} \\ $$$${V}={volume}\:{of}\:{gas}\:\left({air}\right) \\ $$$${the}\:{larger}\:{the}\:{pressure},\:{the}\:{smaller} \\ $$$${the}\:{volume}. \\ $$$${PV}={constant} \\ $$
Commented by mr W last updated on 30/Apr/21
Commented by mr W last updated on 30/Apr/21

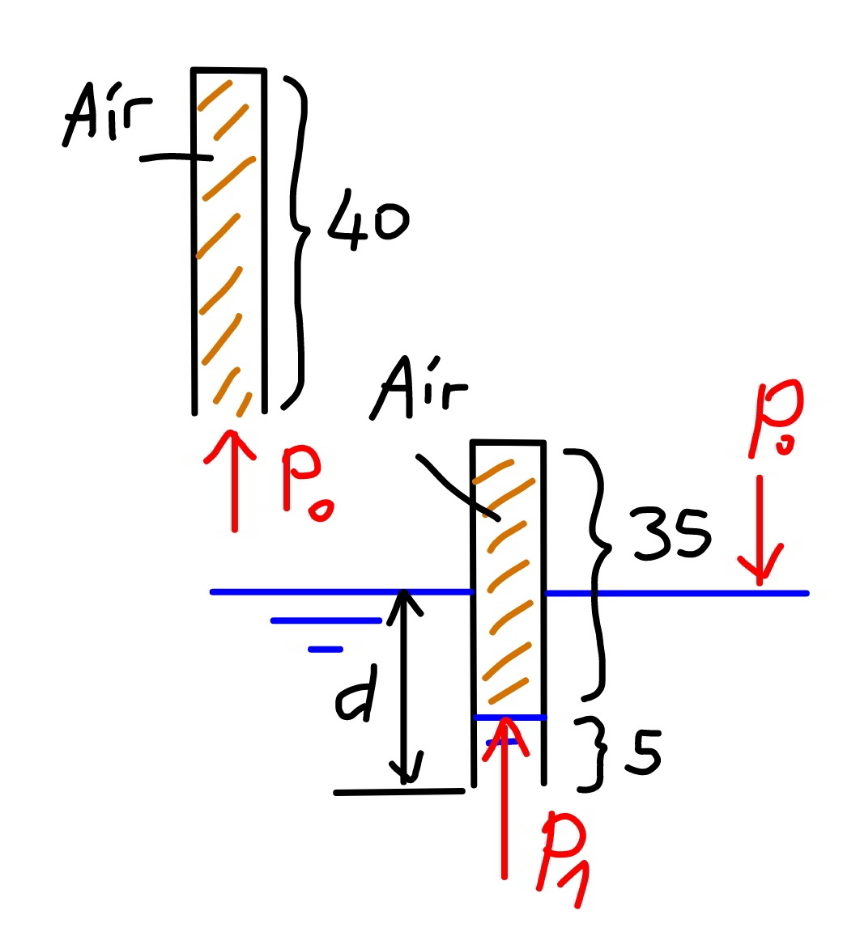
$${say}\:{the}\:{cross}−{section}\:{of}\:{tube}\:{is}\:{A}. \\ $$$${the}\:{volume}\:{of}\:{air}\:{in}\:{tube}\:{under}\: \\ $$$${atmospheric}\:{pressure} \\ $$$${is}\:{V}_{\mathrm{0}} =\mathrm{40}{A},\:{pressure}\:{is}\:{P}_{\mathrm{0}} =\mathrm{77}{cm} \\ $$$${the}\:{volume}\:{of}\:{air}\:{in}\:{tube}\:{in}\:{mercury} \\ $$$${is}\:{V}_{\mathrm{1}} =\mathrm{35}{A},\:{pressure}\:{is}\:{P}_{\mathrm{1}} \:{with} \\ $$$${P}_{\mathrm{1}} ={P}_{\mathrm{0}} +\left({d}−\mathrm{5}\right) \\ $$$$ \\ $$$${P}_{\mathrm{0}} {V}_{\mathrm{0}} ={P}_{\mathrm{1}} {V}_{\mathrm{1}} \\ $$$$\frac{{P}_{\mathrm{1}} }{{P}_{\mathrm{0}} }=\frac{{V}_{\mathrm{0}} }{{V}_{\mathrm{1}} }=\frac{\mathrm{40}}{\mathrm{35}} \\ $$$$\frac{\mathrm{77}+{d}−\mathrm{5}}{\mathrm{77}}=\frac{\mathrm{40}}{\mathrm{35}} \\ $$$${d}=\mathrm{5}+\left(\frac{\mathrm{40}}{\mathrm{35}}−\mathrm{1}\right)×\mathrm{77}=\mathrm{16}\:{cm} \\ $$$$\Rightarrow{the}\:{tube}\:{is}\:\mathrm{16}{cm}\:{immersed}. \\ $$
Commented by I want to learn more last updated on 30/Apr/21
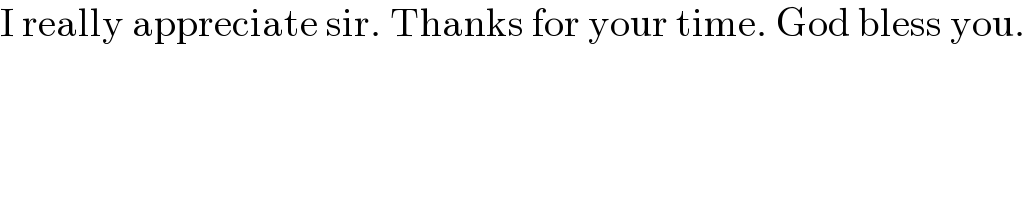
$$\mathrm{I}\:\mathrm{really}\:\mathrm{appreciate}\:\mathrm{sir}.\:\mathrm{Thanks}\:\mathrm{for}\:\mathrm{your}\:\mathrm{time}.\:\mathrm{God}\:\mathrm{bless}\:\mathrm{you}. \\ $$
