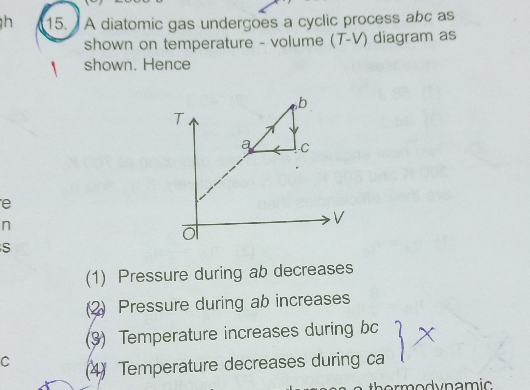
Question Number 75203 by jagannath.02 last updated on 08/Dec/19
Answered by Rio Michael last updated on 08/Dec/19
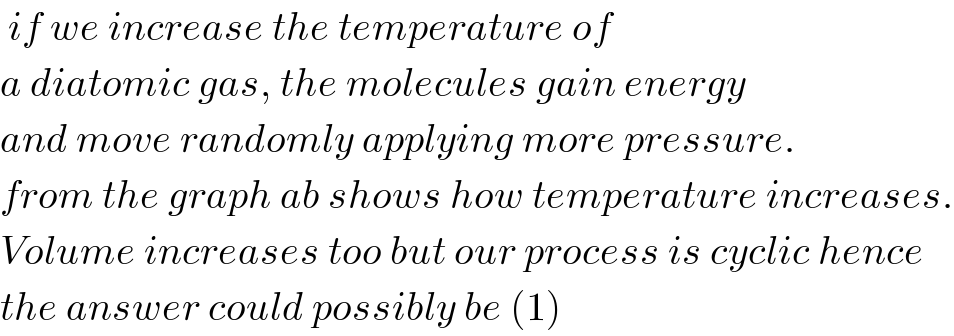
$$\:{if}\:{we}\:{increase}\:{the}\:{temperature}\:{of}\: \\ $$$${a}\:{diatomic}\:{gas},\:{the}\:{molecules}\:{gain}\:{energy} \\ $$$${and}\:{move}\:{randomly}\:{applying}\:{more}\:{pressure}. \\ $$$${from}\:{the}\:{graph}\:{ab}\:{shows}\:{how}\:{temperature}\:{increases}. \\ $$$${Volume}\:{increases}\:{too}\:{but}\:{our}\:{process}\:{is}\:{cyclic}\:{hence} \\ $$$${the}\:{answer}\:{could}\:{possibly}\:{be}\:\left(\mathrm{1}\right) \\ $$
