Question Number 62037 by amingolkar20@gmail.com last updated on 14/Jun/19
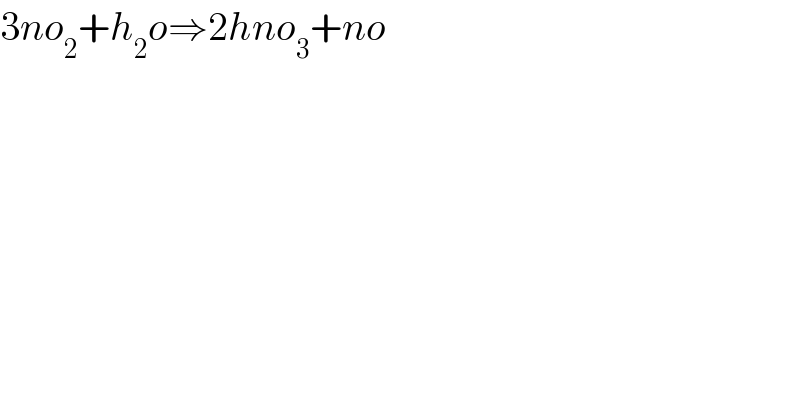
$$\mathrm{3}{no}_{\mathrm{2}} +{h}_{\mathrm{2}} {o}\Rightarrow\mathrm{2}{hno}_{\mathrm{3}} +{no} \\ $$$$ \\ $$
Answered by aleks041103 last updated on 17/Jun/19
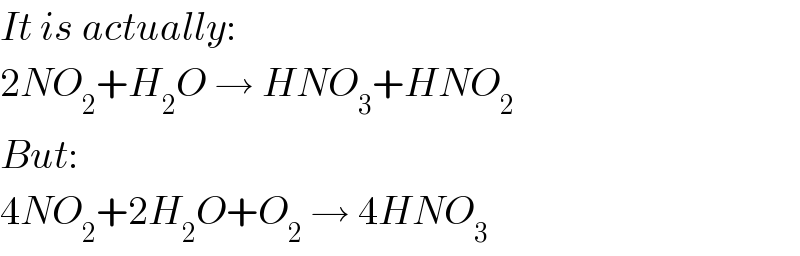
$${It}\:{is}\:{actually}: \\ $$$$\mathrm{2}{NO}_{\mathrm{2}} +{H}_{\mathrm{2}} {O}\:\rightarrow\:{HNO}_{\mathrm{3}} +{HNO}_{\mathrm{2}} \\ $$$${But}: \\ $$$$\mathrm{4}{NO}_{\mathrm{2}} +\mathrm{2}{H}_{\mathrm{2}} {O}+{O}_{\mathrm{2}} \:\rightarrow\:\mathrm{4}{HNO}_{\mathrm{3}} \\ $$
Commented by aleks041103 last updated on 17/Jun/19
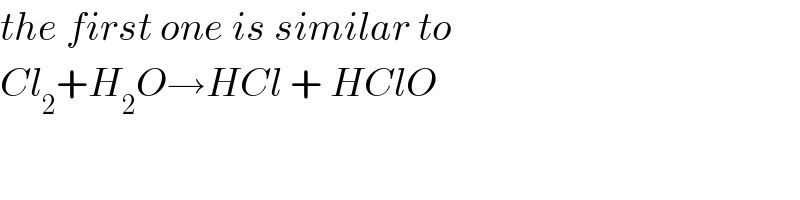
$${the}\:{first}\:{one}\:{is}\:{similar}\:{to} \\ $$$${Cl}_{\mathrm{2}} +{H}_{\mathrm{2}} {O}\rightarrow{HCl}\:+\:{HClO} \\ $$
