Question Number 18411 by Tinkutara last updated on 20/Jul/17
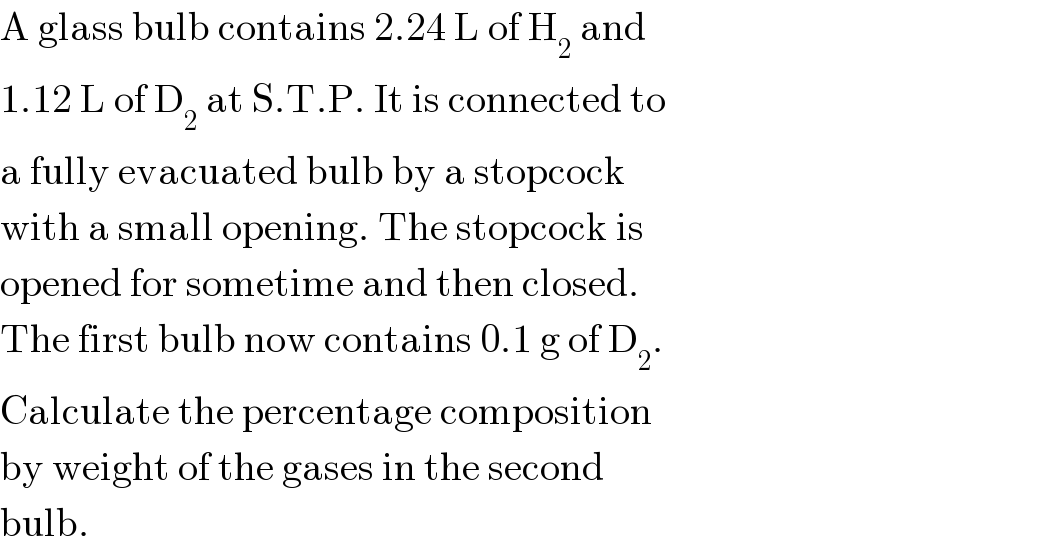
$$\mathrm{A}\:\mathrm{glass}\:\mathrm{bulb}\:\mathrm{contains}\:\mathrm{2}.\mathrm{24}\:\mathrm{L}\:\mathrm{of}\:\mathrm{H}_{\mathrm{2}} \:\mathrm{and} \\ $$$$\mathrm{1}.\mathrm{12}\:\mathrm{L}\:\mathrm{of}\:\mathrm{D}_{\mathrm{2}} \:\mathrm{at}\:\mathrm{S}.\mathrm{T}.\mathrm{P}.\:\mathrm{It}\:\mathrm{is}\:\mathrm{connected}\:\mathrm{to} \\ $$$$\mathrm{a}\:\mathrm{fully}\:\mathrm{evacuated}\:\mathrm{bulb}\:\mathrm{by}\:\mathrm{a}\:\mathrm{stopcock} \\ $$$$\mathrm{with}\:\mathrm{a}\:\mathrm{small}\:\mathrm{opening}.\:\mathrm{The}\:\mathrm{stopcock}\:\mathrm{is} \\ $$$$\mathrm{opened}\:\mathrm{for}\:\mathrm{sometime}\:\mathrm{and}\:\mathrm{then}\:\mathrm{closed}. \\ $$$$\mathrm{The}\:\mathrm{first}\:\mathrm{bulb}\:\mathrm{now}\:\mathrm{contains}\:\mathrm{0}.\mathrm{1}\:\mathrm{g}\:\mathrm{of}\:\mathrm{D}_{\mathrm{2}} . \\ $$$$\mathrm{Calculate}\:\mathrm{the}\:\mathrm{percentage}\:\mathrm{composition} \\ $$$$\mathrm{by}\:\mathrm{weight}\:\mathrm{of}\:\mathrm{the}\:\mathrm{gases}\:\mathrm{in}\:\mathrm{the}\:\mathrm{second} \\ $$$$\mathrm{bulb}. \\ $$
Answered by Tinkutara last updated on 09/Sep/17

