Question Number 155190 by joki last updated on 26/Sep/21
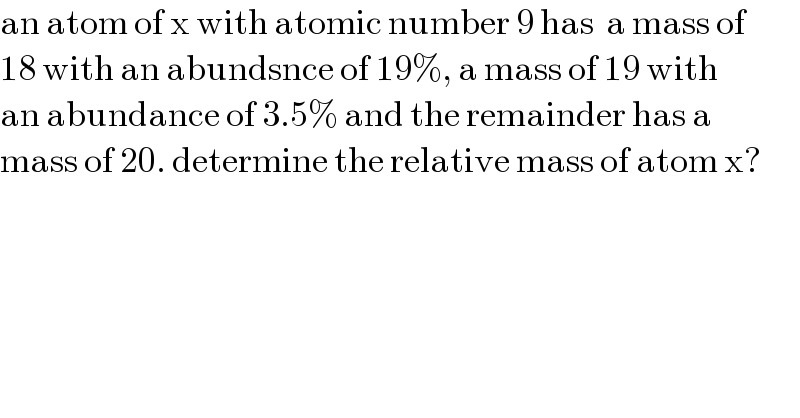
$$\mathrm{an}\:\mathrm{atom}\:\mathrm{of}\:\mathrm{x}\:\mathrm{with}\:\mathrm{atomic}\:\mathrm{number}\:\mathrm{9}\:\mathrm{has}\:\:\mathrm{a}\:\mathrm{mass}\:\mathrm{of} \\ $$$$\mathrm{18}\:\mathrm{with}\:\mathrm{an}\:\mathrm{abundsnce}\:\mathrm{of}\:\mathrm{19\%},\:\mathrm{a}\:\mathrm{mass}\:\mathrm{of}\:\mathrm{19}\:\mathrm{with} \\ $$$$\mathrm{an}\:\mathrm{abundance}\:\mathrm{of}\:\mathrm{3}.\mathrm{5\%}\:\mathrm{and}\:\mathrm{the}\:\mathrm{remainder}\:\mathrm{has}\:\mathrm{a}\: \\ $$$$\mathrm{mass}\:\mathrm{of}\:\mathrm{20}.\:\mathrm{determine}\:\mathrm{the}\:\mathrm{relative}\:\mathrm{mass}\:\mathrm{of}\:\mathrm{atom}\:\mathrm{x}? \\ $$
Answered by physicstutes last updated on 26/Sep/21
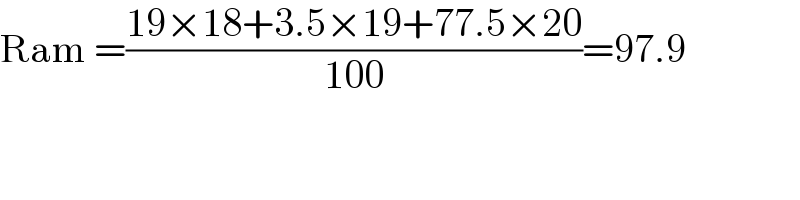
$$\mathrm{Ram}\:=\frac{\mathrm{19}×\mathrm{18}+\mathrm{3}.\mathrm{5}×\mathrm{19}+\mathrm{77}.\mathrm{5}×\mathrm{20}}{\mathrm{100}}=\mathrm{97}.\mathrm{9} \\ $$
