Question Number 104367 by Study last updated on 21/Jul/20

$${CH}_{\mathrm{3}} −{CH}_{\mathrm{2}} −{CH}_{\mathrm{2}} −{CH}_{\mathrm{2}} {Cl}+{KOH}\rightarrow \\ $$$${help}\:{me} \\ $$
Answered by 1549442205PVT last updated on 21/Jul/20
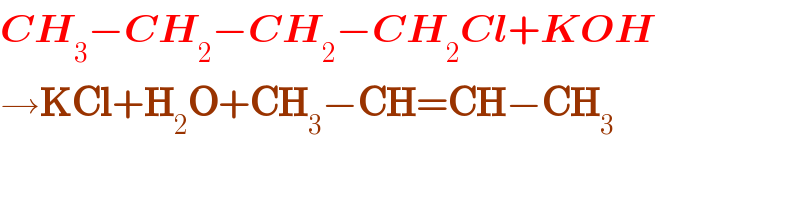
$$\boldsymbol{{CH}}_{\mathrm{3}} −\boldsymbol{{CH}}_{\mathrm{2}} −\boldsymbol{{CH}}_{\mathrm{2}} −\boldsymbol{{CH}}_{\mathrm{2}} \boldsymbol{{Cl}}+\boldsymbol{{KOH}} \\ $$$$\rightarrow\boldsymbol{\mathrm{KCl}}+\boldsymbol{\mathrm{H}}_{\mathrm{2}} \boldsymbol{\mathrm{O}}+\boldsymbol{\mathrm{CH}}_{\mathrm{3}} −\boldsymbol{\mathrm{CH}}=\boldsymbol{\mathrm{CH}}−\boldsymbol{\mathrm{CH}}_{\mathrm{3}} \\ $$
