Question Number 104368 by Study last updated on 21/Jul/20
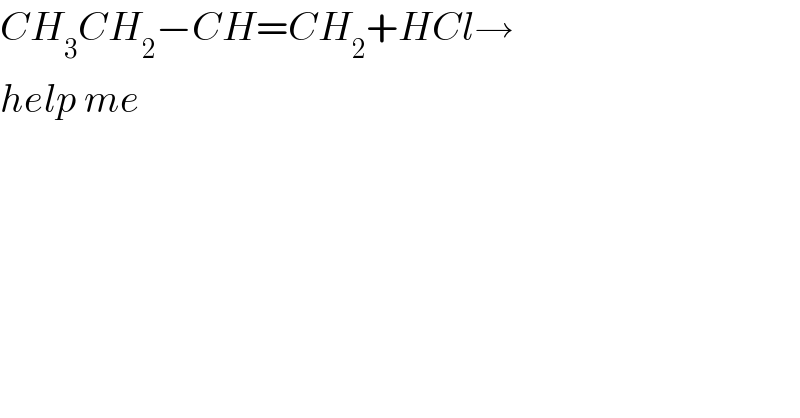
$${CH}_{\mathrm{3}} {CH}_{\mathrm{2}} −{CH}={CH}_{\mathrm{2}} +{HCl}\rightarrow \\ $$$${help}\:{me} \\ $$
Answered by 1549442205PVT last updated on 21/Jul/20
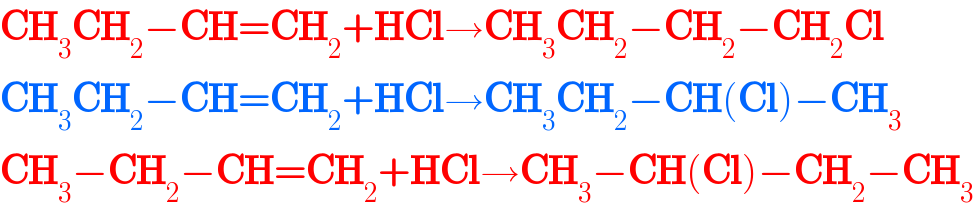
$$\boldsymbol{\mathrm{CH}}_{\mathrm{3}} \boldsymbol{\mathrm{CH}}_{\mathrm{2}} −\boldsymbol{\mathrm{CH}}=\boldsymbol{\mathrm{CH}}_{\mathrm{2}} +\boldsymbol{\mathrm{HCl}}\rightarrow\boldsymbol{\mathrm{CH}}_{\mathrm{3}} \boldsymbol{\mathrm{CH}}_{\mathrm{2}} −\boldsymbol{\mathrm{CH}}_{\mathrm{2}} −\boldsymbol{\mathrm{CH}}_{\mathrm{2}} \boldsymbol{\mathrm{Cl}} \\ $$$$\boldsymbol{\mathrm{CH}}_{\mathrm{3}} \boldsymbol{\mathrm{CH}}_{\mathrm{2}} −\boldsymbol{\mathrm{CH}}=\boldsymbol{\mathrm{CH}}_{\mathrm{2}} +\boldsymbol{\mathrm{HCl}}\rightarrow\boldsymbol{\mathrm{CH}}_{\mathrm{3}} \boldsymbol{\mathrm{CH}}_{\mathrm{2}} −\boldsymbol{\mathrm{CH}}\left(\boldsymbol{\mathrm{Cl}}\right)−\boldsymbol{\mathrm{CH}}_{\mathrm{3}} \\ $$$$\boldsymbol{\mathrm{CH}}_{\mathrm{3}} −\boldsymbol{\mathrm{CH}}_{\mathrm{2}} −\boldsymbol{\mathrm{CH}}=\boldsymbol{\mathrm{CH}}_{\mathrm{2}} +\boldsymbol{\mathrm{HCl}}\rightarrow\boldsymbol{\mathrm{CH}}_{\mathrm{3}} −\boldsymbol{\mathrm{CH}}\left(\boldsymbol{\mathrm{Cl}}\right)−\boldsymbol{\mathrm{CH}}_{\mathrm{2}} −\boldsymbol{\mathrm{CH}}_{\mathrm{3}} \\ $$
Commented by Study last updated on 21/Jul/20
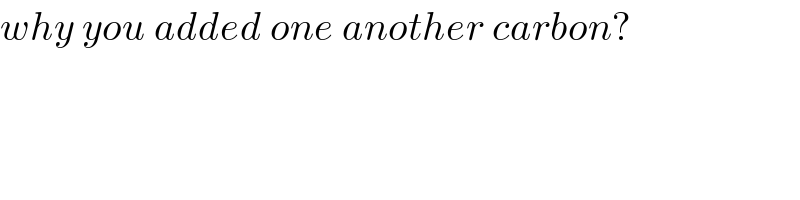
$${why}\:{you}\:{added}\:{one}\:{another}\:{carbon}? \\ $$
Commented by 1549442205PVT last updated on 21/Jul/20
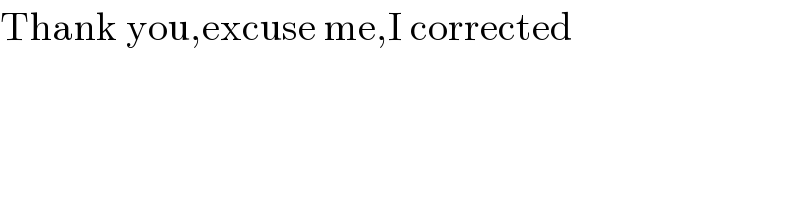
$$\mathrm{Thank}\:\mathrm{you},\mathrm{excuse}\:\mathrm{me},\mathrm{I}\:\mathrm{corrected}\: \\ $$
