Question Number 24436 by Tinkutara last updated on 17/Nov/17
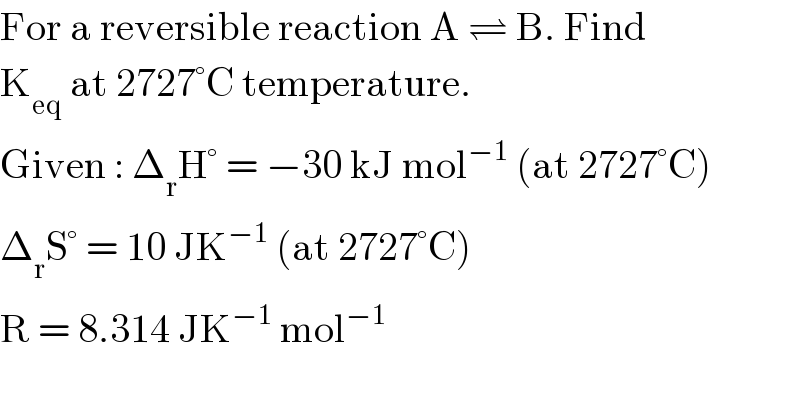
$$\mathrm{For}\:\mathrm{a}\:\mathrm{reversible}\:\mathrm{reaction}\:\mathrm{A}\:\rightleftharpoons\:\mathrm{B}.\:\mathrm{Find} \\ $$$$\mathrm{K}_{\mathrm{eq}} \:\mathrm{at}\:\mathrm{2727}°\mathrm{C}\:\mathrm{temperature}. \\ $$$$\mathrm{Given}\::\:\Delta_{\mathrm{r}} \mathrm{H}°\:=\:−\mathrm{30}\:\mathrm{kJ}\:\mathrm{mol}^{−\mathrm{1}} \:\left(\mathrm{at}\:\mathrm{2727}°\mathrm{C}\right) \\ $$$$\Delta_{\mathrm{r}} \mathrm{S}°\:=\:\mathrm{10}\:\mathrm{JK}^{−\mathrm{1}} \:\left(\mathrm{at}\:\mathrm{2727}°\mathrm{C}\right) \\ $$$$\mathrm{R}\:=\:\mathrm{8}.\mathrm{314}\:\mathrm{JK}^{−\mathrm{1}} \:\mathrm{mol}^{−\mathrm{1}} \\ $$
