Question Number 22594 by Tinkutara last updated on 20/Oct/17

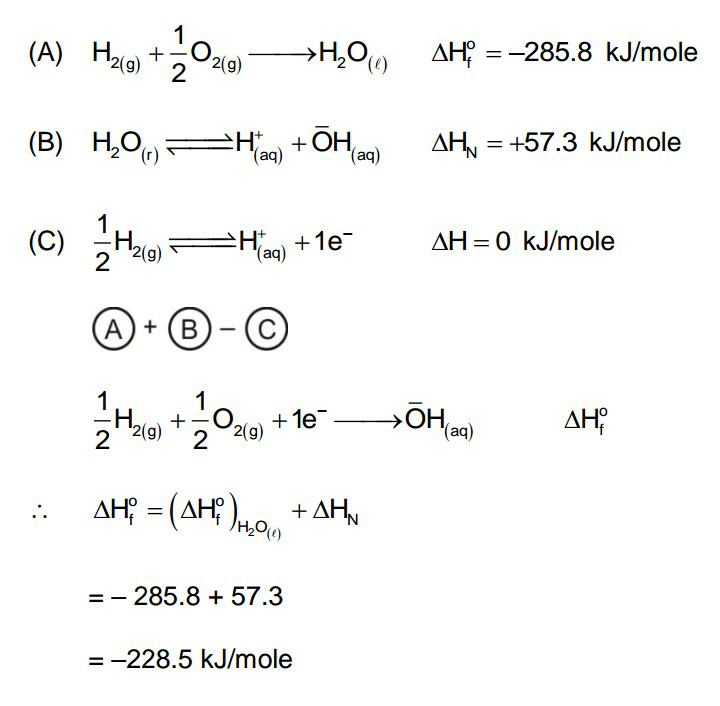
$$\Delta\mathrm{H}_{\mathrm{f}} ^{\mathrm{o}} \:\mathrm{of}\:\mathrm{water}\:\mathrm{is}\:−\mathrm{285}.\mathrm{8}\:\mathrm{kJ}\:\mathrm{mol}^{−\mathrm{1}} .\:\mathrm{If} \\ $$$$\mathrm{enthalpy}\:\mathrm{of}\:\mathrm{neutralization}\:\mathrm{of}\:\mathrm{monoacid} \\ $$$$\mathrm{strong}\:\mathrm{base}\:\mathrm{is}\:−\mathrm{57}.\mathrm{3}\:\mathrm{kJ}\:\mathrm{mol}^{−\mathrm{1}} ,\:\Delta\mathrm{H}_{\mathrm{f}} ^{\mathrm{o}} \:\mathrm{of} \\ $$$$\mathrm{OH}^{−} \:\mathrm{ion}\:\mathrm{will}\:\mathrm{be} \\ $$
Answered by Tinkutara last updated on 23/Oct/17