Question Number 32845 by Rio Mike last updated on 03/Apr/18
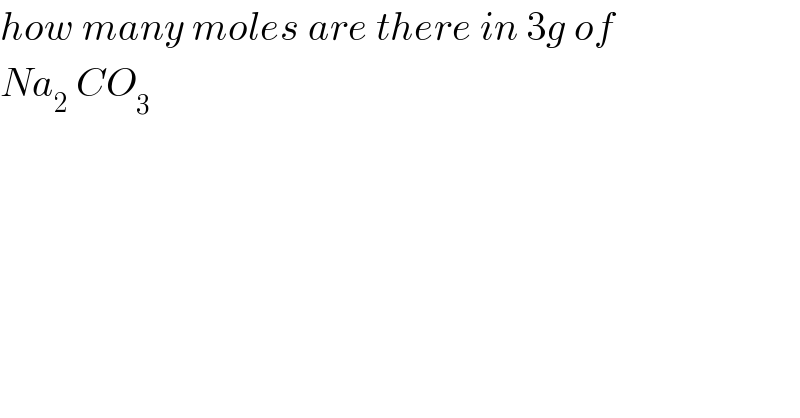
$${how}\:{many}\:{moles}\:{are}\:{there}\:{in}\:\mathrm{3}{g}\:{of} \\ $$$${Na}_{\mathrm{2}_{} } {CO}_{\mathrm{3}} \\ $$
Answered by Joel578 last updated on 04/Apr/18
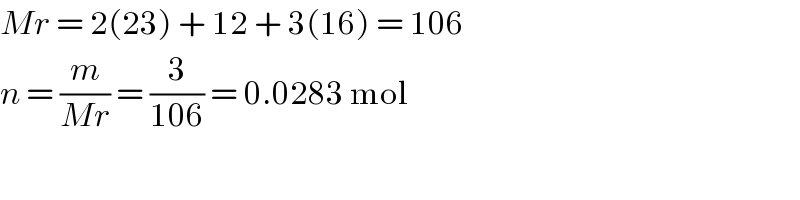
$${Mr}\:=\:\mathrm{2}\left(\mathrm{23}\right)\:+\:\mathrm{12}\:+\:\mathrm{3}\left(\mathrm{16}\right)\:=\:\mathrm{106} \\ $$$${n}\:=\:\frac{{m}}{{Mr}}\:=\:\frac{\mathrm{3}}{\mathrm{106}}\:=\:\mathrm{0}.\mathrm{0283}\:\mathrm{mol} \\ $$
