Question Number 42261 by Necxx last updated on 21/Aug/18
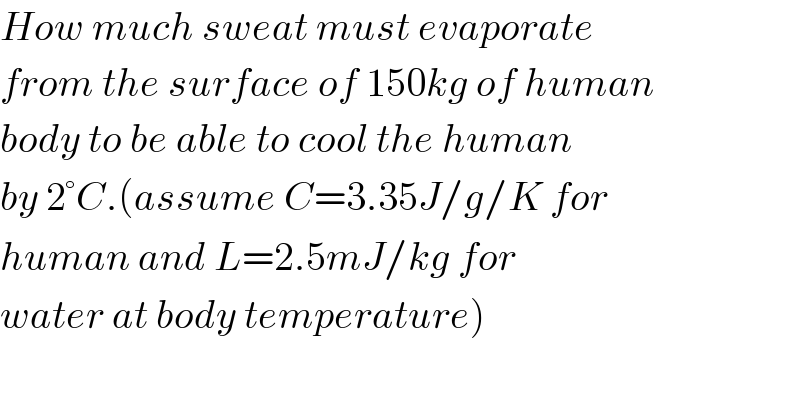
$${How}\:{much}\:{sweat}\:{must}\:{evaporate} \\ $$$${from}\:{the}\:{surface}\:{of}\:\mathrm{150}{kg}\:{of}\:{human} \\ $$$${body}\:{to}\:{be}\:{able}\:{to}\:{cool}\:{the}\:{human} \\ $$$${by}\:\mathrm{2}°{C}.\left({assume}\:{C}=\mathrm{3}.\mathrm{35}{J}/{g}/{K}\:{for}\right. \\ $$$${human}\:{and}\:{L}=\mathrm{2}.\mathrm{5}{mJ}/{kg}\:{for} \\ $$$$\left.{water}\:{at}\:{body}\:{temperature}\right) \\ $$
Commented by Necxx last updated on 21/Aug/18

$${please}\:{help} \\ $$
Answered by tanmay.chaudhury50@gmail.com last updated on 21/Aug/18
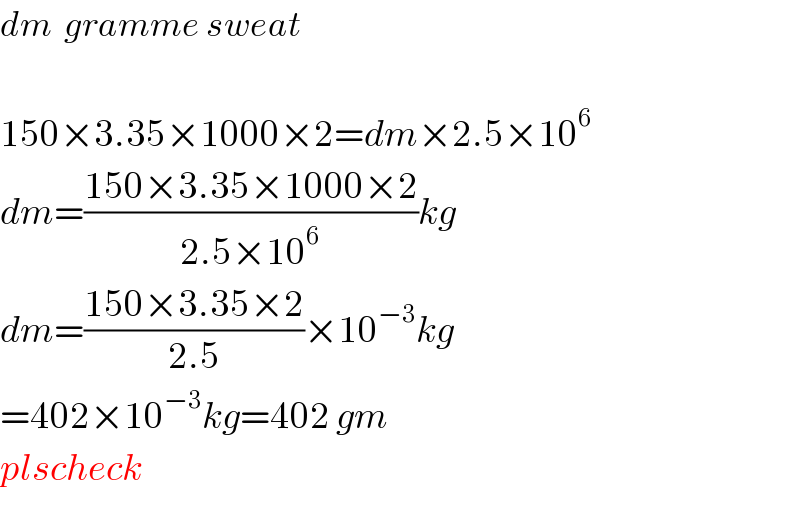
$${dm}\:\:{gramme}\:{sweat} \\ $$$$ \\ $$$$\mathrm{150}×\mathrm{3}.\mathrm{35}×\mathrm{1000}×\mathrm{2}={dm}×\mathrm{2}.\mathrm{5}×\mathrm{10}^{\mathrm{6}} \\ $$$${dm}=\frac{\mathrm{150}×\mathrm{3}.\mathrm{35}×\mathrm{1000}×\mathrm{2}}{\mathrm{2}.\mathrm{5}×\mathrm{10}^{\mathrm{6}} }{kg} \\ $$$${dm}=\frac{\mathrm{150}×\mathrm{3}.\mathrm{35}×\mathrm{2}}{\mathrm{2}.\mathrm{5}}×\mathrm{10}^{−\mathrm{3}} {kg} \\ $$$$=\mathrm{402}×\mathrm{10}^{−\mathrm{3}} {kg}=\mathrm{402}\:{gm} \\ $$$${plscheck} \\ $$
Commented by Necxx last updated on 21/Aug/18
$${Yes}\:{boss}.{It}'{s}\:{correct}.{Thank}\:{you}\:{sir}. \\ $$
