Question Number 15906 by Tinkutara last updated on 15/Jun/17

$$\mathrm{If}\:\lambda_{\mathrm{1}} \:\mathrm{and}\:\lambda_{\mathrm{2}} \:\mathrm{are}\:\mathrm{respectively}\:\mathrm{the} \\ $$$$\mathrm{wavelengths}\:\mathrm{of}\:\mathrm{the}\:\mathrm{series}\:\mathrm{limit}\:\mathrm{of}\:\mathrm{Lyman} \\ $$$$\mathrm{and}\:\mathrm{Balmer}\:\mathrm{series}\:\mathrm{of}\:\mathrm{Hydrogen}\:\mathrm{atom}, \\ $$$$\mathrm{then}\:\mathrm{the}\:\mathrm{wavelength}\:\mathrm{of}\:\mathrm{the}\:\mathrm{first}\:\mathrm{line}\:\mathrm{of} \\ $$$$\mathrm{the}\:\mathrm{Lyman}\:\mathrm{series}\:\mathrm{of}\:\mathrm{the}\:\mathrm{H}-\mathrm{atom}\:\mathrm{is} \\ $$$$\left(\mathrm{1}\right)\:\lambda_{\mathrm{1}} \:−\:\lambda_{\mathrm{2}} \\ $$$$\left(\mathrm{2}\right)\:\sqrt{\lambda_{\mathrm{1}} \lambda_{\mathrm{2}} } \\ $$$$\left(\mathrm{3}\right)\:\frac{\lambda_{\mathrm{2}} \:−\:\lambda_{\mathrm{1}} }{\lambda_{\mathrm{1}} \lambda_{\mathrm{2}} } \\ $$$$\left(\mathrm{4}\right)\:\frac{\lambda_{\mathrm{1}} \lambda_{\mathrm{2}} }{\lambda_{\mathrm{2}} \:−\:\lambda_{\mathrm{1}} } \\ $$
Answered by ajfour last updated on 15/Jun/17
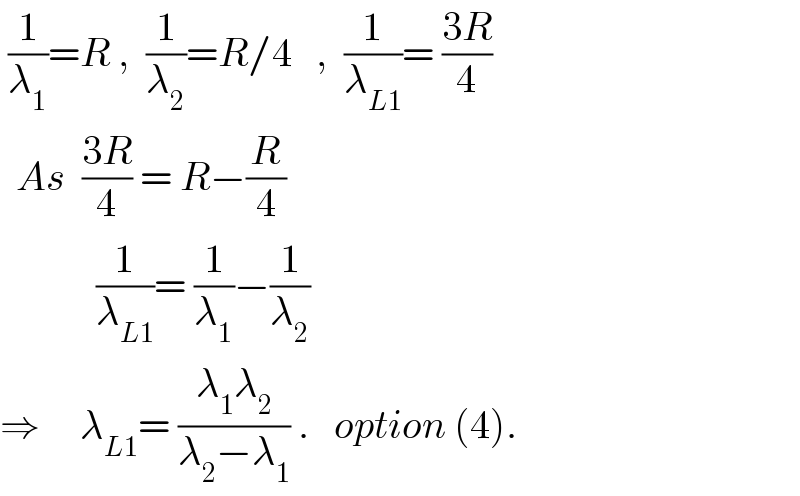
$$\:\frac{\mathrm{1}}{\lambda_{\mathrm{1}} }={R}\:,\:\:\frac{\mathrm{1}}{\lambda_{\mathrm{2}} }={R}/\mathrm{4}\:\:\:,\:\:\frac{\mathrm{1}}{\lambda_{{L}\mathrm{1}} }=\:\frac{\mathrm{3}{R}}{\mathrm{4}} \\ $$$$\:\:{As}\:\:\frac{\mathrm{3}{R}}{\mathrm{4}}\:=\:{R}−\frac{{R}}{\mathrm{4}} \\ $$$$\:\:\:\:\:\:\:\:\:\:\:\:\frac{\mathrm{1}}{\lambda_{{L}\mathrm{1}} }=\:\frac{\mathrm{1}}{\lambda_{\mathrm{1}} }−\frac{\mathrm{1}}{\lambda_{\mathrm{2}} } \\ $$$$\Rightarrow\:\:\:\:\:\lambda_{{L}\mathrm{1}} =\:\frac{\lambda_{\mathrm{1}} \lambda_{\mathrm{2}} }{\lambda_{\mathrm{2}} −\lambda_{\mathrm{1}} }\:.\:\:\:{option}\:\left(\mathrm{4}\right). \\ $$
Commented by Tinkutara last updated on 15/Jun/17

$$\mathrm{Thanks}\:\mathrm{Sir}! \\ $$
