Question Number 155561 by peter frank last updated on 02/Oct/21

Answered by mr W last updated on 02/Oct/21

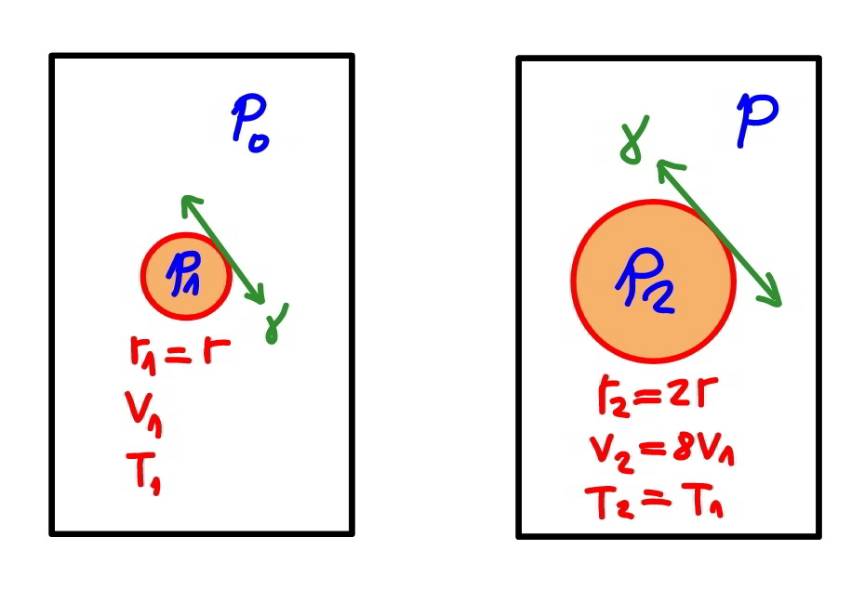
$${p}_{\mathrm{1}} ={pressure}\:{inside}\:{bubble}\:{at}\:{radius}\:{r} \\ $$$${p}_{\mathrm{2}} ={pressure}\:{inside}\:{bubble}\:{at}\:{radius}\:\mathrm{2}{r} \\ $$$${p}_{\mathrm{1}} −{p}_{\mathrm{0}} =\frac{\mathrm{4}\gamma}{{r}}\:\Rightarrow{p}_{\mathrm{1}} ={p}_{\mathrm{0}} +\frac{\mathrm{4}\gamma}{{r}} \\ $$$${p}_{\mathrm{2}} −{p}=\frac{\mathrm{4}\gamma}{\mathrm{2}{r}}\:\Rightarrow{p}_{\mathrm{2}} ={p}+\frac{\mathrm{2}\gamma}{{r}} \\ $$$$\frac{{p}_{\mathrm{2}} }{{p}_{\mathrm{1}} }=\frac{{V}_{\mathrm{1}} }{{V}_{\mathrm{2}} }=\left(\frac{{r}}{\mathrm{2}{r}}\right)^{\mathrm{3}} =\frac{\mathrm{1}}{\mathrm{8}} \\ $$$${p}+\frac{\mathrm{2}\gamma}{{r}}=\frac{\mathrm{1}}{\mathrm{8}}\left({p}_{\mathrm{0}} +\frac{\mathrm{4}\gamma}{{r}}\right) \\ $$$${p}=\frac{\mathrm{1}}{\mathrm{8}}\left({p}_{\mathrm{0}} +\frac{\mathrm{4}\gamma}{{r}}\right)−\frac{\mathrm{2}\gamma}{{r}} \\ $$$$\Rightarrow{p}=\frac{\mathrm{1}}{\mathrm{8}}\left({p}_{\mathrm{0}} −\frac{\mathrm{12}\gamma}{{r}}\right) \\ $$
Commented by mr W last updated on 02/Oct/21
Commented by peter frank last updated on 02/Oct/21

$$\mathrm{great}\:\mathrm{sir}\:;\mathrm{thanks} \\ $$
Commented by Tawa11 last updated on 02/Oct/21

$$\mathrm{great}\:\mathrm{sir} \\ $$
