Question Number 178009 by Spillover last updated on 12/Oct/22

Commented by Beginner last updated on 12/Oct/22

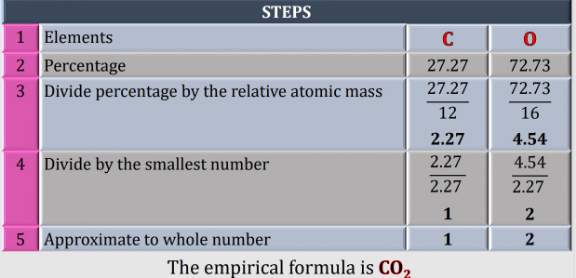
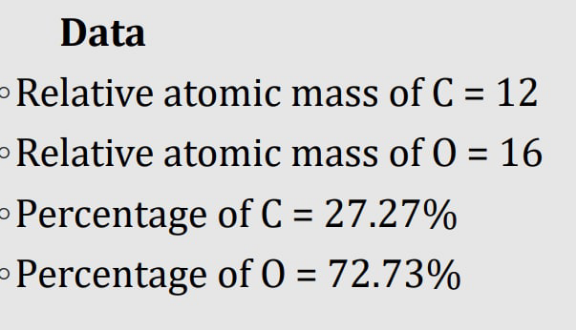
$${oxygen}\:{should}\:{be}\:\mathrm{16} \\ $$
Commented by Spillover last updated on 12/Oct/22
![yes your right.thank you for reminding me.[C=12 O=16]](https://www.tinkutara.com/question/Q178017.png)
$$\mathrm{yes}\:\mathrm{your}\:\mathrm{right}.\mathrm{thank}\:\mathrm{you}\:\mathrm{for} \\ $$$$\mathrm{reminding}\:\mathrm{me}.\left[\mathrm{C}=\mathrm{12}\:\:\mathrm{O}=\mathrm{16}\right] \\ $$
Answered by a.lgnaoui last updated on 12/Oct/22
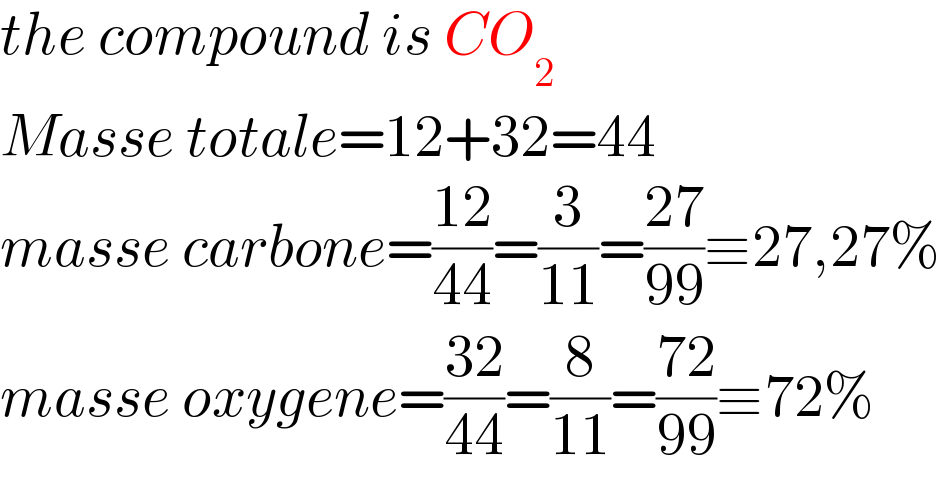
$${the}\:{compound}\:{is}\:{CO}_{\mathrm{2}} \\ $$$${Masse}\:{totale}=\mathrm{12}+\mathrm{32}=\mathrm{44} \\ $$$${masse}\:{carbone}=\frac{\mathrm{12}}{\mathrm{44}}=\frac{\mathrm{3}}{\mathrm{11}}=\frac{\mathrm{27}}{\mathrm{99}}\equiv\mathrm{27},\mathrm{27\%} \\ $$$${masse}\:{oxygene}=\frac{\mathrm{32}}{\mathrm{44}}=\frac{\mathrm{8}}{\mathrm{11}}=\frac{\mathrm{72}}{\mathrm{99}}\equiv\mathrm{72\%} \\ $$
Commented by Spillover last updated on 12/Oct/22

$$\mathrm{thank}\:\mathrm{you} \\ $$
Answered by Spillover last updated on 12/Oct/22
Answered by Spillover last updated on 12/Oct/22