Question Number 28076 by Tinkutara last updated on 20/Jan/18
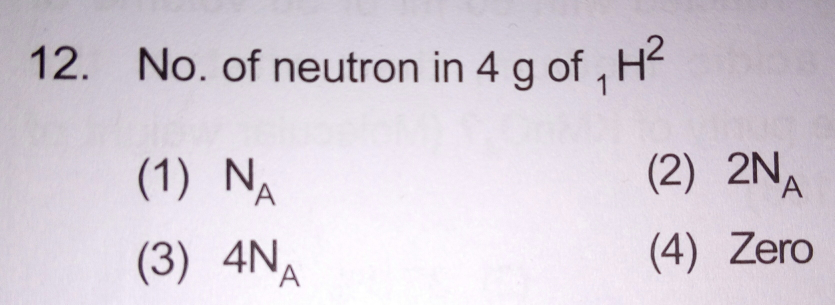
Commented by Tinkutara last updated on 20/Jan/18
Answer given is 3rd. Is the answer correct?
Answered by ajfour last updated on 20/Jan/18
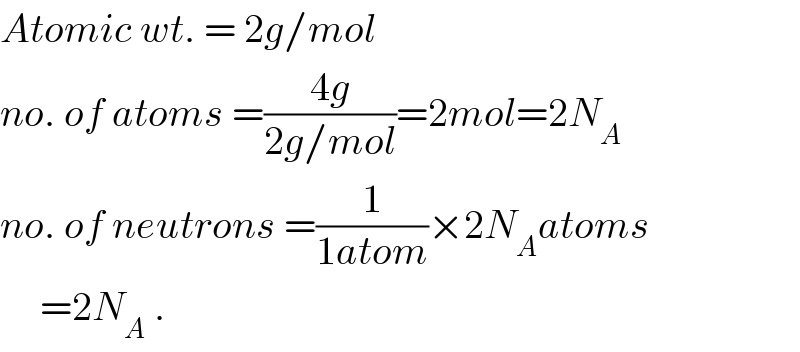
$${Atomic}\:{wt}.\:=\:\mathrm{2}{g}/{mol} \\ $$$${no}.\:{of}\:{atoms}\:=\frac{\mathrm{4}{g}}{\mathrm{2}{g}/{mol}}=\mathrm{2}{mol}=\mathrm{2}{N}_{{A}} \\ $$$${no}.\:{of}\:{neutrons}\:=\frac{\mathrm{1}}{\mathrm{1}{atom}}×\mathrm{2}{N}_{{A}} {atoms} \\ $$$$\:\:\:\:\:=\mathrm{2}{N}_{{A}} \:. \\ $$
Commented by Tinkutara last updated on 20/Jan/18
Yes this is the same I got thanks but I wanted to confirm the answer.
