Question Number 28626 by Cheyboy last updated on 28/Jan/18

Commented by Tinkutara last updated on 28/Jan/18
Which one?
Commented by Cheyboy last updated on 28/Jan/18

$${both}\:{of}\:{them}\:{sir} \\ $$
Commented by Tinkutara last updated on 28/Jan/18
Please check the answers:
a. 12.063 M and 10.137 molality
b. 8.33×10^(-17)
Commented by Cheyboy last updated on 28/Jan/18
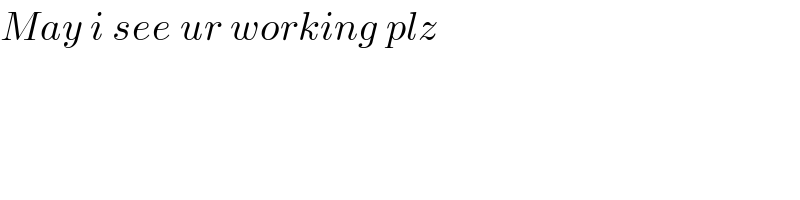
$${May}\:{i}\:{see}\:{ur}\:{working}\:{plz} \\ $$
Commented by Tinkutara last updated on 28/Jan/18
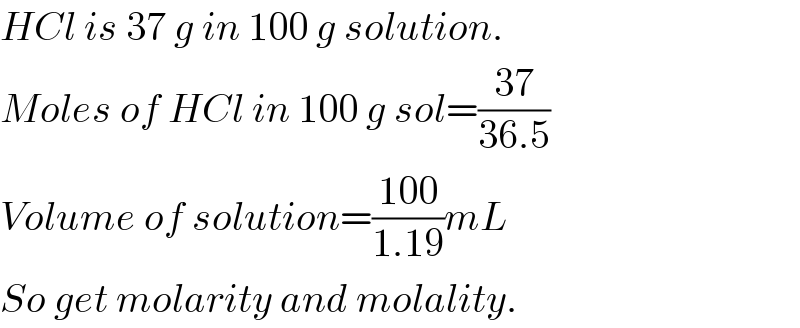
$${HCl}\:{is}\:\mathrm{37}\:{g}\:{in}\:\mathrm{100}\:{g}\:{solution}. \\ $$$${Moles}\:{of}\:{HCl}\:{in}\:\mathrm{100}\:{g}\:{sol}=\frac{\mathrm{37}}{\mathrm{36}.\mathrm{5}} \\ $$$${Volume}\:{of}\:{solution}=\frac{\mathrm{100}}{\mathrm{1}.\mathrm{19}}{mL} \\ $$$${So}\:{get}\:{molarity}\:{and}\:{molality}. \\ $$
Commented by Cheyboy last updated on 28/Jan/18
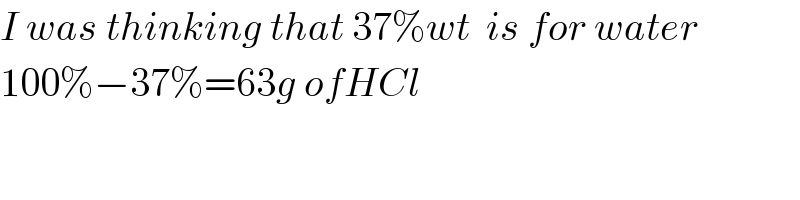
$${I}\:{was}\:{thinking}\:{that}\:\mathrm{37\%}{wt}\:\:{is}\:{for}\:{water} \\ $$$$\mathrm{100\%}−\mathrm{37\%}=\mathrm{63}{g}\:{ofHCl} \\ $$
Commented by Cheyboy last updated on 28/Jan/18
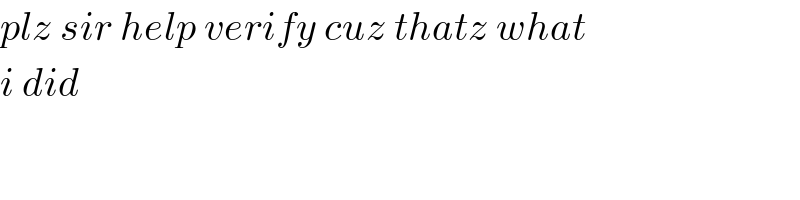
$${plz}\:{sir}\:{help}\:{verify}\:{cuz}\:{thatz}\:{what} \\ $$$${i}\:{did} \\ $$
Commented by Tinkutara last updated on 28/Jan/18
37%(w/w) solution of HCl always means 37 g of HCl is present in 100 g solution.
Do you have answer keys?
Commented by Cheyboy last updated on 28/Jan/18
$${no}\:{sir}\:{i}\:{dnt}\:{knw}\:{the}\:{answer}\:{it}\:{was} \\ $$$${a}\:{test}\:{given}\:{to}\:{us}.{thank}\:{for}\:{d} \\ $$$${explaination} \\ $$$$ \\ $$
