Question Number 30626 by Tinkutara last updated on 23/Feb/18

Commented by Cheyboy last updated on 23/Feb/18
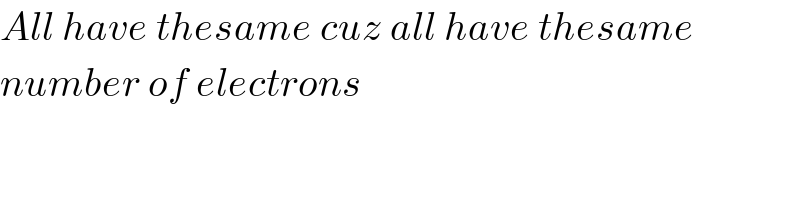
$${All}\:{have}\:{thesame}\:{cuz}\:{all}\:{have}\:{thesame} \\ $$$${number}\:{of}\:{electrons} \\ $$
Commented by rahul 19 last updated on 24/Feb/18
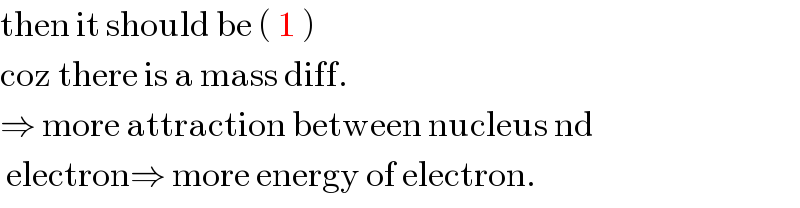
$$\mathrm{then}\:\mathrm{it}\:\mathrm{should}\:\mathrm{be}\:\left(\:\mathrm{1}\:\right) \\ $$$$\mathrm{coz}\:\mathrm{there}\:\mathrm{is}\:\mathrm{a}\:\mathrm{mass}\:\mathrm{diff}.\: \\ $$$$\Rightarrow\:\mathrm{more}\:\mathrm{attraction}\:\mathrm{between}\:\mathrm{nucleus}\:\mathrm{nd}\: \\ $$$$\:\mathrm{electron}\Rightarrow\:\mathrm{more}\:\mathrm{energy}\:\mathrm{of}\:\mathrm{electron}. \\ $$
Commented by Tinkutara last updated on 24/Feb/18
@rahul 19 1st option is correct but all the isotopes have 1 electron only then why it makes a difference?
Commented by rahul 19 last updated on 24/Feb/18

$$\mathrm{suppose}\:\mathrm{u}\:\mathrm{r}\:\mathrm{given}\:\mathrm{3}\:\mathrm{species}\:\mathrm{which}\:\mathrm{are} \\ $$$$\mathrm{isoelectronic}\:,\:\mathrm{then}\:\mathrm{u}\:\mathrm{mean}\:\mathrm{all}\:\mathrm{electrons} \\ $$$$\mathrm{will}\:\mathrm{have}\:\mathrm{same}\:\mathrm{energy}? \\ $$
Commented by rahul 19 last updated on 24/Feb/18

$$\mathrm{even}\:\mathrm{the}\:\mathrm{total}\:\mathrm{energy}\:\mathrm{will}\:\mathrm{not}\:\mathrm{be}\:\mathrm{same}! \\ $$$$\mathrm{and}\:\mathrm{most}\:\mathrm{importantly}\:\mathrm{electrons}\:\mathrm{are}\: \\ $$$$\mathrm{revolving}\:\mathrm{around}\:\mathrm{centre}\:\mathrm{of}\:\mathrm{mass}\:\mathrm{of}\:\mathrm{that}\:\mathrm{atom}. \\ $$
Commented by Tinkutara last updated on 01/Mar/18
Based on which formula we can say this?
