Question Number 51289 by rahul 19 last updated on 25/Dec/18
Commented by Tinkutara last updated on 26/Dec/18
Ohh sorry! Thanks!
Commented by rahul 19 last updated on 26/Dec/18
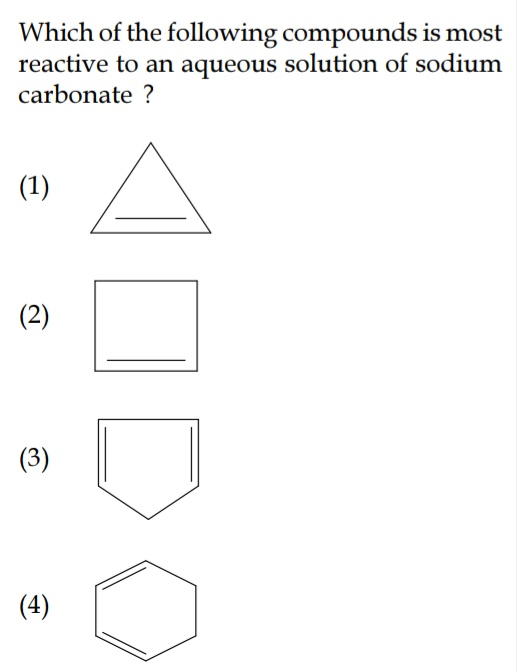
when negative charge is formed in option 3 , it will become AROMATIC. ����
(this is Exact reason).
Commented by Tinkutara last updated on 26/Dec/18
Na2CO3 extracts acidic hydrogen so when negative charge is formed in option 3, it has maximum number of resonating structures. (I am not sure though)
Commented by Tinkutara last updated on 26/Dec/18
But 1st also becomes aromatic with negative charge on the top vertex.
Commented by rahul 19 last updated on 26/Dec/18
Nahh, it will become Anti-Aromatic.
It would have become aromatic if it were to have +ve charge at top vertex! ��
Answered by afachri last updated on 25/Dec/18

$$\left(\mathrm{1}\right)\:\mathrm{siklopropena} \\ $$
Commented by rahul 19 last updated on 25/Dec/18

$${Ans}\:{is}\:\left(\mathrm{3}\right)\:. \\ $$
Commented by afachri last updated on 25/Dec/18

$$\mathrm{uhm}\:\mathrm{i}\:\mathrm{was}\:\mathrm{wrong}\:\mathrm{Sir}.\:\mathrm{so}\:\mathrm{that}\:\mathrm{the}\:\mathrm{point}\:\mathrm{is},\: \\ $$$$\mathrm{cause}\:\mathrm{the}\:\left(\mathrm{3}\right)\:\mathrm{compunds}\:\mathrm{has}\:\mathrm{more}\:\mathrm{double} \\ $$$$\mathrm{bonds}\:\mathrm{than}\:\mathrm{the}\:\mathrm{others}\:\left(\mathrm{cyclohexena}\:\mathrm{excluded}\right. \\ $$$$\left.\mathrm{cause}\:\mathrm{it}'\mathrm{s}\:\mathrm{stable}\right).\:\mathrm{The}\:\mathrm{number}\:\mathrm{of}\:\mathrm{double} \\ $$$$\mathrm{bonds}\:\mathrm{affects}\:\mathrm{reactivity}\:\mathrm{compounds}. \\ $$$$\mathrm{thanks}\:\mathrm{Sir}\:\mathrm{for}\:\mathrm{sharing}\:\mathrm{this}\:\mathrm{question}. \\ $$
