Question Number 54825 by Tawa1 last updated on 12/Feb/19
Answered by tanmay.chaudhury50@gmail.com last updated on 12/Feb/19
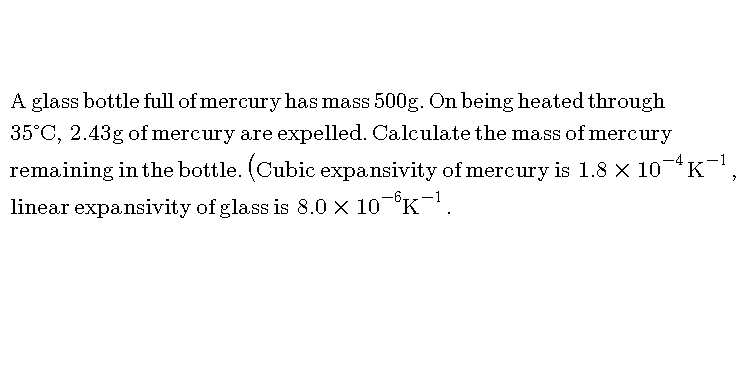
![v_0 =volume of glass bottle before heating v_0 (1+γ_g △T)=volume of glass bottle after heating v_0 (1+γ_m △T)=volume of mercury after heating m_e =mass of mercury expelled d_0 =density of mercury before heating mass of mercury remain same... v_0 (1+γ_m △T)((d_0 /(1+γ_m △T)))=v_0 (1+γ_g △T)×((d_0 /(1+γ_m △T)))+m_e v_0 ((d_0 /(1+γ_m △T)))(γ_m −γ_g )△T=m_e ((m_0 /(1+γ_m △T)))(γ_m −γ_g )△T=m_e m_0 =((m_e (1+γ_m △T))/((γ_m −γ_g )△T)) m_0 =((m_e (1+γ_m △T))/((γ_m −3α_g )△T)) required answer is m_0 −m_e m_e [((1+γ_m △T)/((γ_m −3α_g )△T))−1] pls check...and put values...](https://www.tinkutara.com/question/Q54828.png)
$${v}_{\mathrm{0}} ={volume}\:{of}\:{glass}\:{bottle}\:{before}\:{heating} \\ $$$${v}_{\mathrm{0}} \left(\mathrm{1}+\gamma_{{g}} \bigtriangleup{T}\right)={volume}\:{of}\:{glass}\:{bottle}\:{after}\:{heating} \\ $$$${v}_{\mathrm{0}} \left(\mathrm{1}+\gamma_{{m}} \bigtriangleup{T}\right)={volume}\:{of}\:{mercury}\:{after}\:{heating} \\ $$$${m}_{{e}} ={mass}\:{of}\:{mercury}\:{expelled} \\ $$$${d}_{\mathrm{0}} ={density}\:{of}\:{mercury}\:{before}\:{heating} \\ $$$${mass}\:{of}\:{mercury}\:{remain}\:{same}… \\ $$$${v}_{\mathrm{0}} \left(\mathrm{1}+\gamma_{{m}} \bigtriangleup{T}\right)\left(\frac{{d}_{\mathrm{0}} }{\mathrm{1}+\gamma_{{m}} \bigtriangleup{T}}\right)={v}_{\mathrm{0}} \left(\mathrm{1}+\gamma_{{g}} \bigtriangleup{T}\right)×\left(\frac{{d}_{\mathrm{0}} }{\mathrm{1}+\gamma_{{m}} \bigtriangleup{T}}\right)+{m}_{{e}} \\ $$$${v}_{\mathrm{0}} \left(\frac{{d}_{\mathrm{0}} }{\mathrm{1}+\gamma_{{m}} \bigtriangleup{T}}\right)\left(\gamma_{{m}} −\gamma_{{g}} \right)\bigtriangleup{T}={m}_{{e}} \\ $$$$\left(\frac{{m}_{\mathrm{0}} }{\mathrm{1}+\gamma_{{m}} \bigtriangleup{T}}\right)\left(\gamma_{{m}} −\gamma_{{g}} \right)\bigtriangleup{T}={m}_{{e}} \\ $$$${m}_{\mathrm{0}} =\frac{{m}_{{e}} \left(\mathrm{1}+\gamma_{{m}} \bigtriangleup{T}\right)}{\left(\gamma_{{m}} −\gamma_{{g}} \right)\bigtriangleup{T}} \\ $$$${m}_{\mathrm{0}} =\frac{{m}_{{e}} \left(\mathrm{1}+\gamma_{{m}} \bigtriangleup{T}\right)}{\left(\gamma_{{m}} −\mathrm{3}\alpha_{{g}} \right)\bigtriangleup{T}} \\ $$$${required}\:{answer}\:{is} \\ $$$${m}_{\mathrm{0}} −{m}_{{e}} \\ $$$${m}_{{e}} \left[\frac{\mathrm{1}+\gamma_{{m}} \bigtriangleup{T}}{\left(\gamma_{{m}} −\mathrm{3}\alpha_{{g}} \right)\bigtriangleup{T}}−\mathrm{1}\right] \\ $$$${pls}\:{check}…{and}\:{put}\:{values}… \\ $$$$ \\ $$$$ \\ $$
Commented by Tawa1 last updated on 13/Feb/19
$$\mathrm{God}\:\mathrm{bless}\:\mathrm{you}\:\mathrm{sir} \\ $$
