Question Number 62923 by peter frank last updated on 26/Jun/19

Answered by peter frank last updated on 27/Jun/19
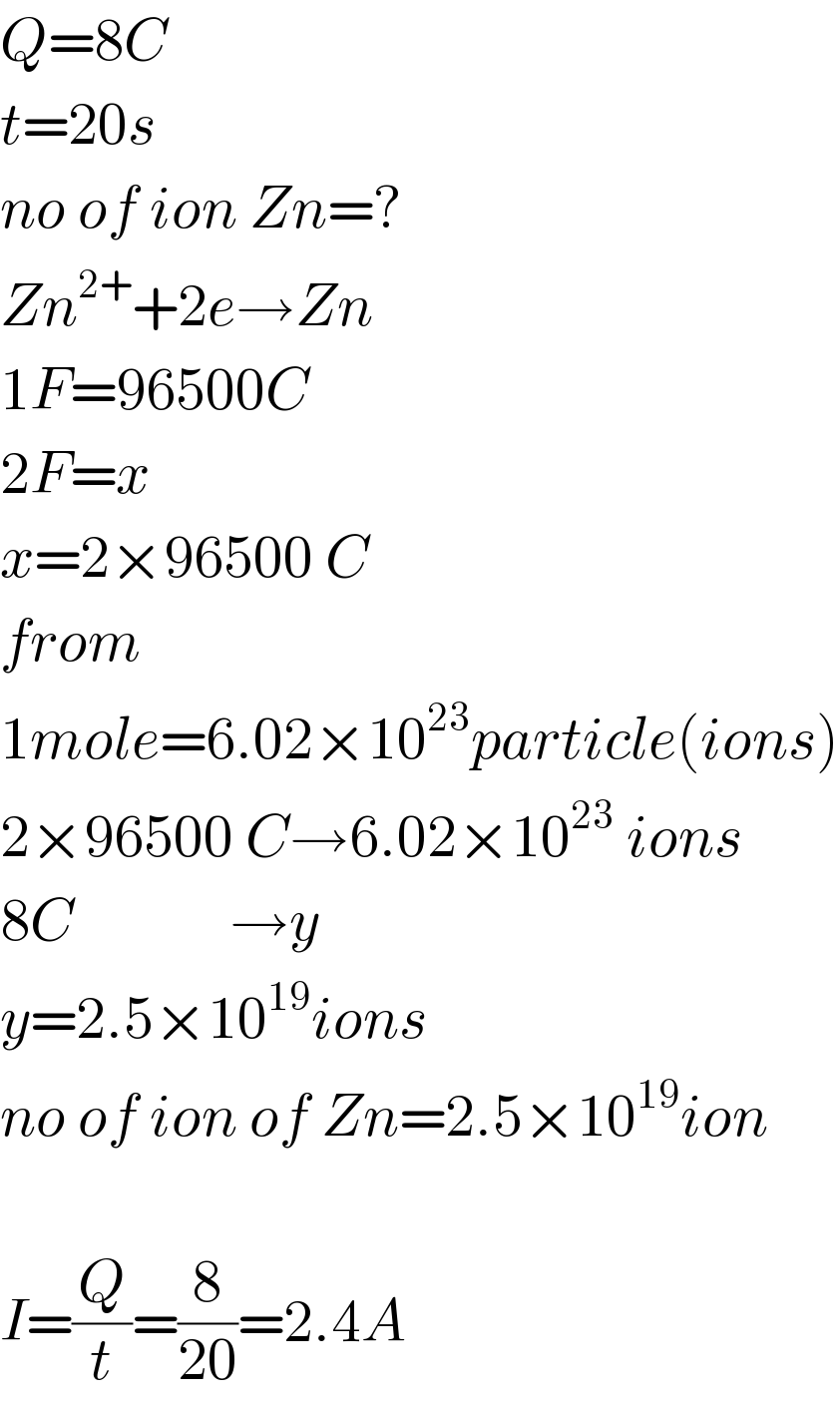
$${Q}=\mathrm{8}{C} \\ $$$${t}=\mathrm{20}{s} \\ $$$${no}\:{of}\:{ion}\:{Zn}=? \\ $$$${Zn}^{\mathrm{2}+} +\mathrm{2}{e}\rightarrow{Zn} \\ $$$$\mathrm{1}{F}=\mathrm{96500}{C} \\ $$$$\mathrm{2}{F}={x} \\ $$$${x}=\mathrm{2}×\mathrm{96500}\:{C} \\ $$$${from} \\ $$$$\mathrm{1}{mole}=\mathrm{6}.\mathrm{02}×\mathrm{10}^{\mathrm{23}} {particle}\left({ions}\right) \\ $$$$\mathrm{2}×\mathrm{96500}\:{C}\rightarrow\mathrm{6}.\mathrm{02}×\mathrm{10}^{\mathrm{23}} \:{ions} \\ $$$$\mathrm{8}{C}\:\:\:\:\:\:\:\:\:\:\:\:\:\rightarrow{y} \\ $$$${y}=\mathrm{2}.\mathrm{5}×\mathrm{10}^{\mathrm{19}} {ions} \\ $$$${no}\:{of}\:{ion}\:{of}\:{Zn}=\mathrm{2}.\mathrm{5}×\mathrm{10}^{\mathrm{19}} {ion} \\ $$$$ \\ $$$${I}=\frac{{Q}}{{t}}=\frac{\mathrm{8}}{\mathrm{20}}=\mathrm{2}.\mathrm{4}{A} \\ $$
Answered by peter frank last updated on 27/Jun/19
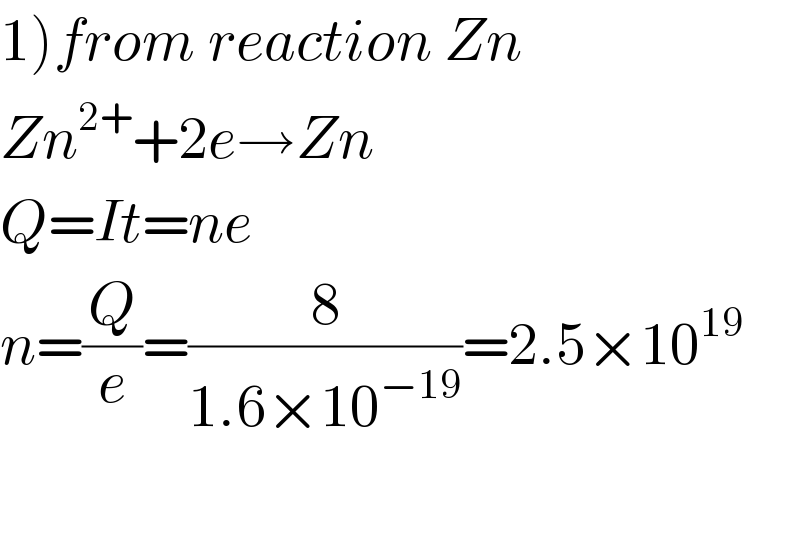
$$\left.\mathrm{1}\right){from}\:{reaction}\:{Zn} \\ $$$${Zn}^{\mathrm{2}+} +\mathrm{2}{e}\rightarrow{Zn} \\ $$$${Q}={It}={ne} \\ $$$${n}=\frac{{Q}}{{e}}=\frac{\mathrm{8}}{\mathrm{1}.\mathrm{6}×\mathrm{10}^{−\mathrm{19}} }=\mathrm{2}.\mathrm{5}×\mathrm{10}^{\mathrm{19}} \\ $$$$ \\ $$
