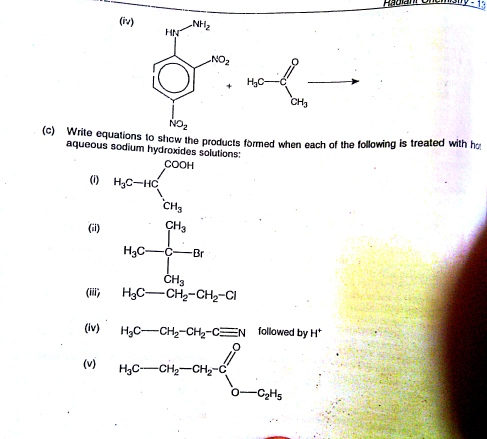
Question Number 92016 by peter frank last updated on 04/May/20
Answered by Rio Michael last updated on 04/May/20
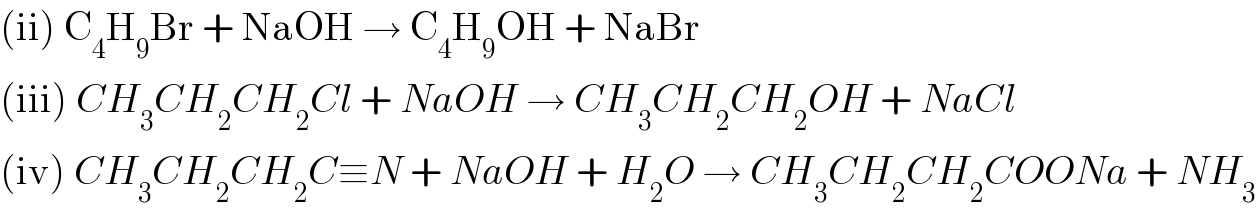
$$\left(\mathrm{ii}\right)\:\mathrm{C}_{\mathrm{4}} \mathrm{H}_{\mathrm{9}} \mathrm{Br}\:+\:\mathrm{NaOH}\:\rightarrow\:\mathrm{C}_{\mathrm{4}} \mathrm{H}_{\mathrm{9}} \mathrm{OH}\:+\:\mathrm{NaBr} \\ $$$$\left(\mathrm{iii}\right)\:{CH}_{\mathrm{3}} {CH}_{\mathrm{2}} {CH}_{\mathrm{2}} {Cl}\:+\:{NaOH}\:\rightarrow\:{CH}_{\mathrm{3}} {CH}_{\mathrm{2}} {CH}_{\mathrm{2}} {OH}\:+\:{NaCl} \\ $$$$\left(\mathrm{iv}\right)\:{CH}_{\mathrm{3}} {CH}_{\mathrm{2}} {CH}_{\mathrm{2}} {C}\equiv{N}\:+\:{NaOH}\:+\:{H}_{\mathrm{2}} {O}\:\rightarrow\:{CH}_{\mathrm{3}} {CH}_{\mathrm{2}} {CH}_{\mathrm{2}} {COONa}\:+\:{NH}_{\mathrm{3}} \\ $$
Commented by peter frank last updated on 10/May/20

$${thank}\:{you} \\ $$
