Question Number 23146 by Tinkutara last updated on 26/Oct/17
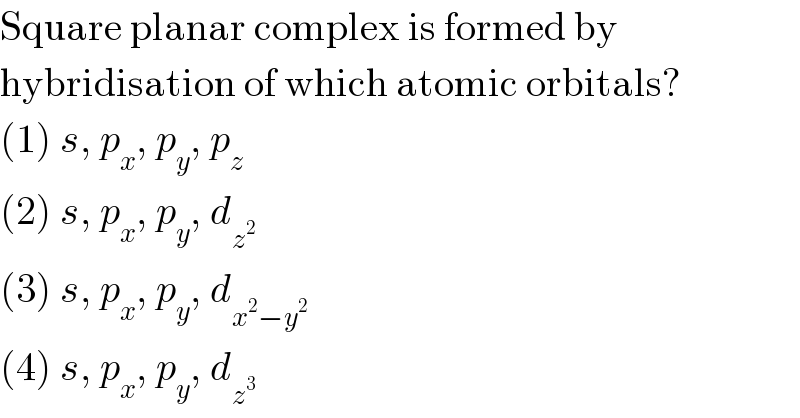
$$\mathrm{Square}\:\mathrm{planar}\:\mathrm{complex}\:\mathrm{is}\:\mathrm{formed}\:\mathrm{by} \\ $$$$\mathrm{hybridisation}\:\mathrm{of}\:\mathrm{which}\:\mathrm{atomic}\:\mathrm{orbitals}? \\ $$$$\left(\mathrm{1}\right)\:{s},\:{p}_{{x}} ,\:{p}_{{y}} ,\:{p}_{{z}} \\ $$$$\left(\mathrm{2}\right)\:{s},\:{p}_{{x}} ,\:{p}_{{y}} ,\:{d}_{{z}^{\mathrm{2}} } \\ $$$$\left(\mathrm{3}\right)\:{s},\:{p}_{{x}} ,\:{p}_{{y}} ,\:{d}_{{x}^{\mathrm{2}} −{y}^{\mathrm{2}} } \\ $$$$\left(\mathrm{4}\right)\:{s},\:{p}_{{x}} ,\:{p}_{{y}} ,\:{d}_{{z}^{\mathrm{3}} } \\ $$
Commented by math solver last updated on 26/Oct/17
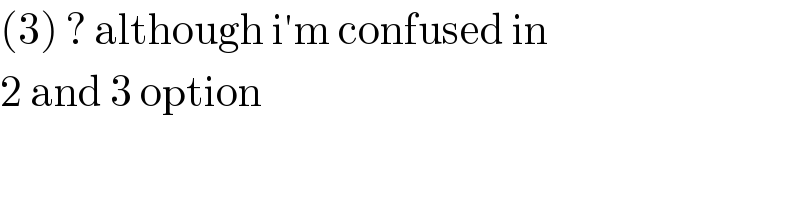
$$\left(\mathrm{3}\right)\:?\:\mathrm{although}\:\mathrm{i}'\mathrm{m}\:\mathrm{confused}\:\mathrm{in} \\ $$$$\mathrm{2}\:\mathrm{and}\:\mathrm{3}\:\mathrm{option} \\ $$$$ \\ $$
Commented by math solver last updated on 26/Oct/17

$$\mathrm{actually}\:\mathrm{square}\:\mathrm{planar}\:\mathrm{compex}\: \\ $$$$\mathrm{has}\:\mathrm{hybridisation}\:\mathrm{dsp}^{\mathrm{2}} . \\ $$$$\mathrm{so}\:\mathrm{going}\:\mathrm{by}\:\mathrm{option}\:\mathrm{only}\:\mathrm{2}\:\mathrm{and}\:\mathrm{3}\: \\ $$$$\mathrm{satisfy}\:\mathrm{and}\:\mathrm{d}_{\mathrm{z}_{} ^{\mathrm{3}} } \:\mathrm{is}\:\mathrm{nothing}…. \\ $$$$ \\ $$
Commented by Tinkutara last updated on 26/Oct/17
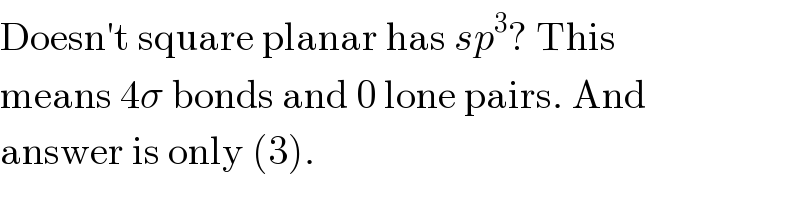
$$\mathrm{Doesn}'\mathrm{t}\:\mathrm{square}\:\mathrm{planar}\:\mathrm{has}\:{sp}^{\mathrm{3}} ?\:\mathrm{This} \\ $$$$\mathrm{means}\:\mathrm{4}\sigma\:\mathrm{bonds}\:\mathrm{and}\:\mathrm{0}\:\mathrm{lone}\:\mathrm{pairs}.\:\mathrm{And} \\ $$$$\mathrm{answer}\:\mathrm{is}\:\mathrm{only}\:\left(\mathrm{3}\right). \\ $$
Commented by math solver last updated on 26/Oct/17

$$\mathrm{square}\:\mathrm{planar}\:\mathrm{and}\:\mathrm{square}\:\mathrm{planar} \\ $$$$\mathrm{complex}\:\mathrm{are}\:\mathrm{different}\:. \\ $$$$\mathrm{square}\:\mathrm{planar}\:\mathrm{has}\:\mathrm{hybridization}\: \\ $$$$\mathrm{sp}^{\mathrm{3}} \mathrm{d}^{\mathrm{2}} .\:\mathrm{eg}:\:\mathrm{XeF}_{\mathrm{4}} \:. \\ $$
Commented by Tinkutara last updated on 27/Oct/17

$$\mathrm{XeF}_{\mathrm{4}} \:\mathrm{has}\:\mathrm{2}\:\mathrm{axial}\:\mathrm{lone}\:\mathrm{pairs}\:\mathrm{also}.\:\mathrm{So}\:\mathrm{why} \\ $$$$\mathrm{this}\:\mathrm{is}\:\mathrm{square}\:\mathrm{planar}?\:\mathrm{It}\:\mathrm{should}\:\mathrm{be} \\ $$$$\mathrm{bipyramidal}. \\ $$
