Question Number 22782 by Tinkutara last updated on 22/Oct/17

$$\mathrm{Successive}\:\mathrm{ionisation}\:\mathrm{energies}\:\mathrm{of} \\ $$$$\mathrm{element}\:\mathrm{is}\:\mathrm{738},\:\mathrm{1450},\:\mathrm{10540},\:\mathrm{13630} \\ $$$$\mathrm{kJ}/\mathrm{mole}\:\mathrm{respectively}.\:\mathrm{The}\:\mathrm{element}\:\mathrm{is} \\ $$$$\left(\mathrm{1}\right)\:\mathrm{Na} \\ $$$$\left(\mathrm{2}\right)\:\mathrm{Mg} \\ $$$$\left(\mathrm{3}\right)\:\mathrm{Al} \\ $$$$\left(\mathrm{4}\right)\:\mathrm{K} \\ $$
Commented by math solver last updated on 22/Oct/17

$${Mg} \\ $$
Commented by math solver last updated on 22/Oct/17
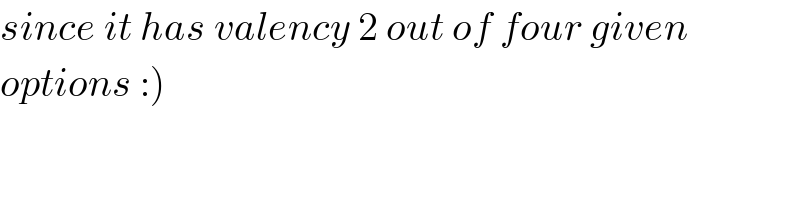
$${since}\:{it}\:{has}\:{valency}\:\mathrm{2}\:{out}\:{of}\:{four}\:{given}\: \\ $$$$\left.{options}\::\right) \\ $$
Commented by Tinkutara last updated on 22/Oct/17
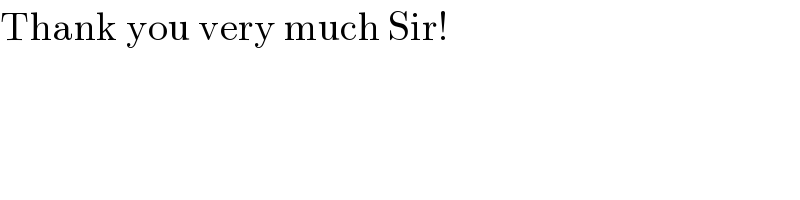
$$\mathrm{Thank}\:\mathrm{you}\:\mathrm{very}\:\mathrm{much}\:\mathrm{Sir}! \\ $$
Commented by math solver last updated on 22/Oct/17

$$\left.{u}\:{r}\:{welcome}\::\right) \\ $$
Commented by Tinkutara last updated on 22/Oct/17
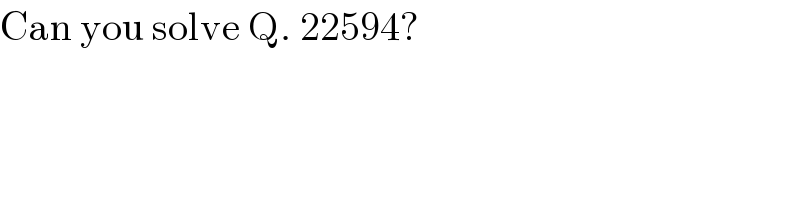
$$\mathrm{Can}\:\mathrm{you}\:\mathrm{solve}\:\mathrm{Q}.\:\mathrm{22594}? \\ $$
Commented by math solver last updated on 22/Oct/17

$${i}\:{haven}'{t}\:{studied}\:{thermo}\:{properly}\:… \\ $$$${and}\:{binomial}\:{i}\:{theorem}\:{i}\:{will}\:{most}\: \\ $$$${probably}\:{study}\:{next}\:{month}…. \\ $$$${so}\:{i}\:{can}'{t}\:{clear}\:{your}\:{doubts}\: \\ $$
