Question Number 22295 by Tinkutara last updated on 14/Oct/17

$$\mathrm{The}\:\mathrm{ionization}\:\mathrm{potential}\:\mathrm{of}\:\mathrm{hydrogen}\:\mathrm{is} \\ $$$$\mathrm{13}.\mathrm{6}\:\mathrm{eV}/\mathrm{mole}.\:\mathrm{Calculate}\:\mathrm{the}\:\mathrm{energy}\:\mathrm{in} \\ $$$$\mathrm{kJ}\:\mathrm{required}\:\mathrm{to}\:\mathrm{produce}\:\mathrm{0}.\mathrm{1}\:\mathrm{mole}\:\mathrm{of}\:\mathrm{H}^{+} \\ $$$$\left.\mathrm{ions}.\:\mathrm{Given},\:\mathrm{1}\:\mathrm{eV}\:=\:\mathrm{96}.\mathrm{49}\:\mathrm{kJ}\:\mathrm{mol}^{−\mathrm{1}} \right) \\ $$
Answered by ajfour last updated on 15/Oct/17
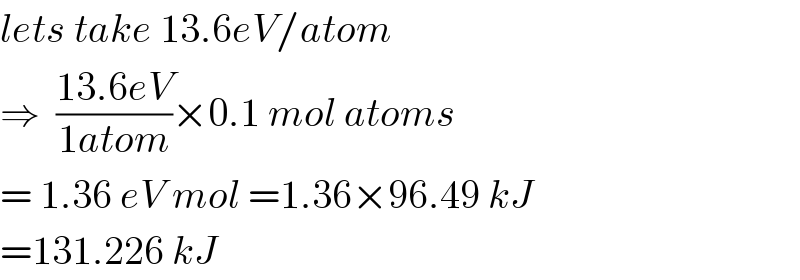
$${lets}\:{take}\:\mathrm{13}.\mathrm{6}{eV}/{atom} \\ $$$$\Rightarrow\:\:\frac{\mathrm{13}.\mathrm{6}{eV}}{\mathrm{1}{atom}}×\mathrm{0}.\mathrm{1}\:{mol}\:{atoms} \\ $$$$=\:\mathrm{1}.\mathrm{36}\:{eV}\:{mol}\:=\mathrm{1}.\mathrm{36}×\mathrm{96}.\mathrm{49}\:{kJ} \\ $$$$=\mathrm{131}.\mathrm{226}\:{kJ}\: \\ $$
Commented by Tinkutara last updated on 15/Oct/17
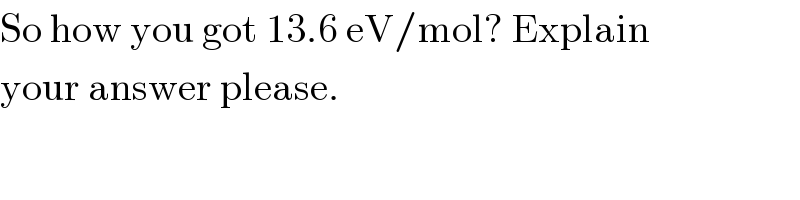
$$\mathrm{So}\:\mathrm{how}\:\mathrm{you}\:\mathrm{got}\:\mathrm{13}.\mathrm{6}\:\mathrm{eV}/\mathrm{mol}?\:\mathrm{Explain} \\ $$$$\mathrm{your}\:\mathrm{answer}\:\mathrm{please}. \\ $$
