Question Number 23135 by Tinkutara last updated on 26/Oct/17
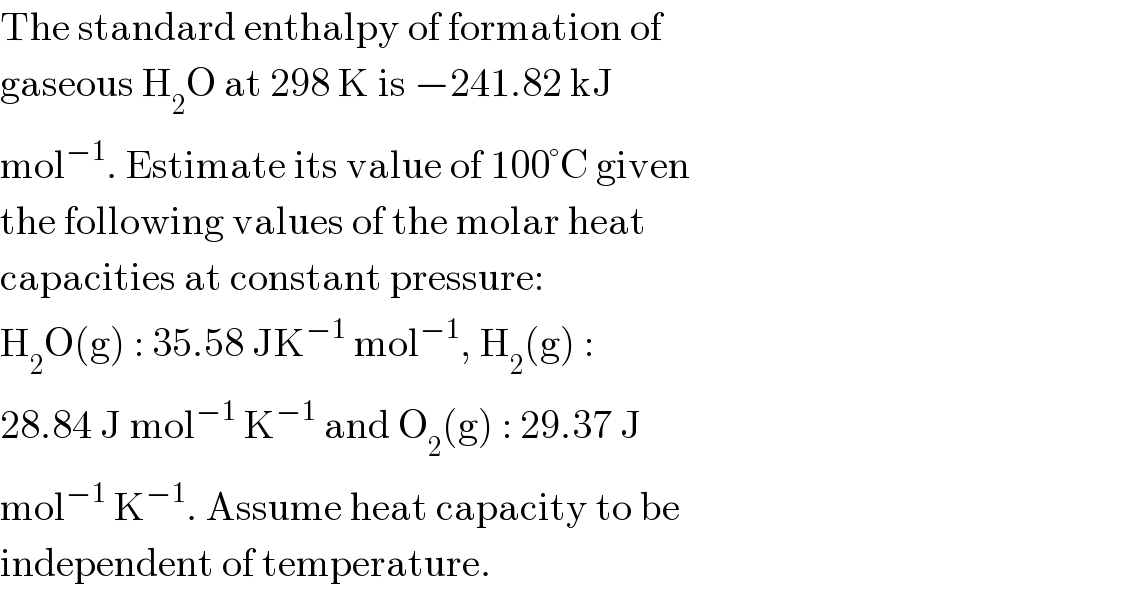
$$\mathrm{The}\:\mathrm{standard}\:\mathrm{enthalpy}\:\mathrm{of}\:\mathrm{formation}\:\mathrm{of} \\ $$$$\mathrm{gaseous}\:\mathrm{H}_{\mathrm{2}} \mathrm{O}\:\mathrm{at}\:\mathrm{298}\:\mathrm{K}\:\mathrm{is}\:−\mathrm{241}.\mathrm{82}\:\mathrm{kJ} \\ $$$$\mathrm{mol}^{−\mathrm{1}} .\:\mathrm{Estimate}\:\mathrm{its}\:\mathrm{value}\:\mathrm{of}\:\mathrm{100}°\mathrm{C}\:\mathrm{given} \\ $$$$\mathrm{the}\:\mathrm{following}\:\mathrm{values}\:\mathrm{of}\:\mathrm{the}\:\mathrm{molar}\:\mathrm{heat} \\ $$$$\mathrm{capacities}\:\mathrm{at}\:\mathrm{constant}\:\mathrm{pressure}: \\ $$$$\mathrm{H}_{\mathrm{2}} \mathrm{O}\left(\mathrm{g}\right)\::\:\mathrm{35}.\mathrm{58}\:\mathrm{JK}^{−\mathrm{1}} \:\mathrm{mol}^{−\mathrm{1}} ,\:\mathrm{H}_{\mathrm{2}} \left(\mathrm{g}\right)\:: \\ $$$$\mathrm{28}.\mathrm{84}\:\mathrm{J}\:\mathrm{mol}^{−\mathrm{1}} \:\mathrm{K}^{−\mathrm{1}} \:\mathrm{and}\:\mathrm{O}_{\mathrm{2}} \left(\mathrm{g}\right)\::\:\mathrm{29}.\mathrm{37}\:\mathrm{J} \\ $$$$\mathrm{mol}^{−\mathrm{1}} \:\mathrm{K}^{−\mathrm{1}} .\:\mathrm{Assume}\:\mathrm{heat}\:\mathrm{capacity}\:\mathrm{to}\:\mathrm{be} \\ $$$$\mathrm{independent}\:\mathrm{of}\:\mathrm{temperature}. \\ $$
Answered by Tinkutara last updated on 28/Oct/17

Commented by Tinkutara last updated on 28/Oct/17

Commented by Tinkutara last updated on 28/Oct/17

Commented by Tinkutara last updated on 28/Oct/17

