Question Number 17179 by tawa tawa last updated on 01/Jul/17

Answered by Tinkutara last updated on 02/Jul/17
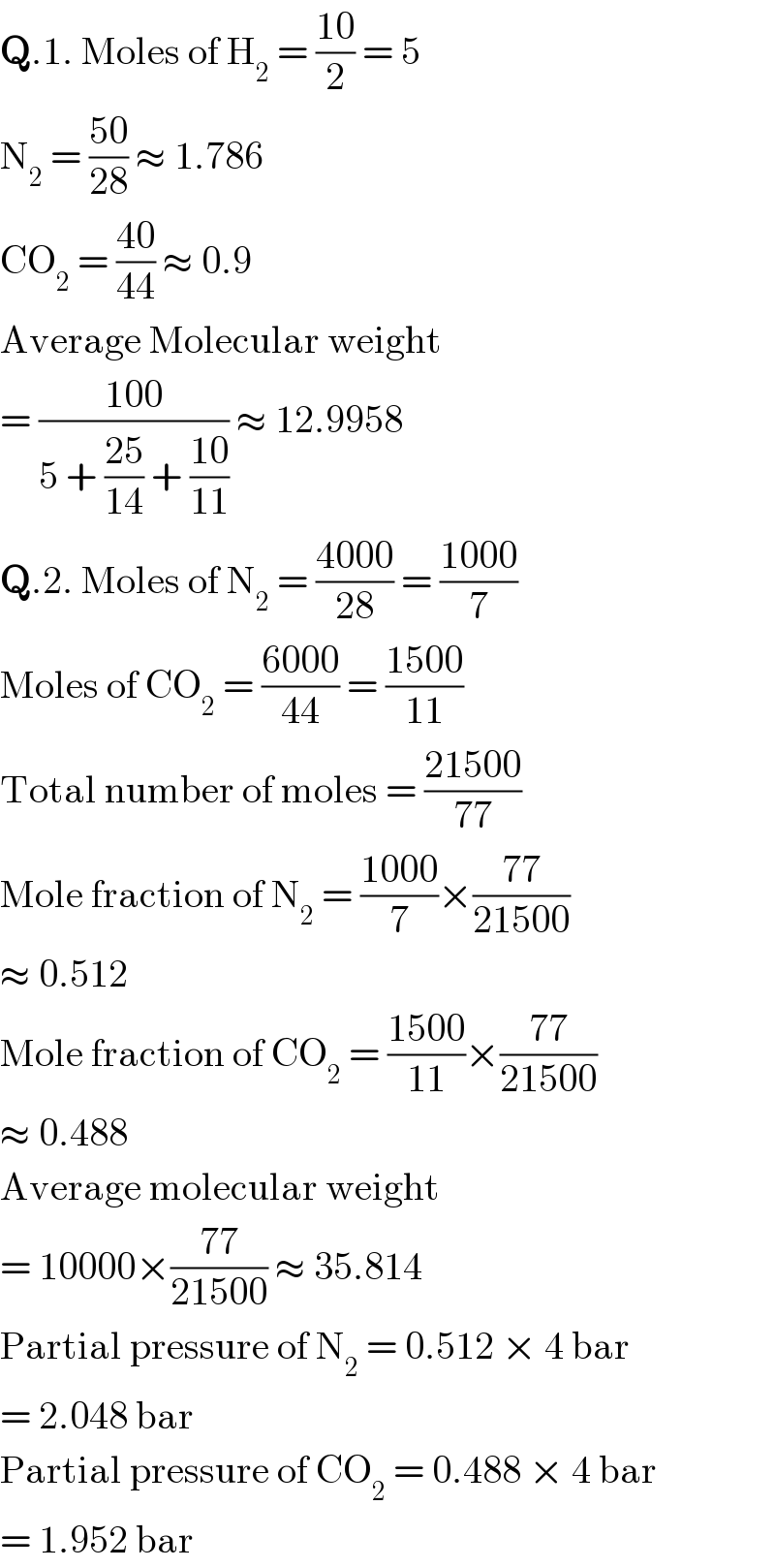
$$\boldsymbol{\mathrm{Q}}.\mathrm{1}.\:\mathrm{Moles}\:\mathrm{of}\:\mathrm{H}_{\mathrm{2}} \:=\:\frac{\mathrm{10}}{\mathrm{2}}\:=\:\mathrm{5} \\ $$$$\mathrm{N}_{\mathrm{2}} \:=\:\frac{\mathrm{50}}{\mathrm{28}}\:\approx\:\mathrm{1}.\mathrm{786} \\ $$$$\mathrm{CO}_{\mathrm{2}} \:=\:\frac{\mathrm{40}}{\mathrm{44}}\:\approx\:\mathrm{0}.\mathrm{9} \\ $$$$\mathrm{Average}\:\mathrm{Molecular}\:\mathrm{weight} \\ $$$$=\:\frac{\mathrm{100}}{\mathrm{5}\:+\:\frac{\mathrm{25}}{\mathrm{14}}\:+\:\frac{\mathrm{10}}{\mathrm{11}}}\:\approx\:\mathrm{12}.\mathrm{9958} \\ $$$$\boldsymbol{\mathrm{Q}}.\mathrm{2}.\:\mathrm{Moles}\:\mathrm{of}\:\mathrm{N}_{\mathrm{2}} \:=\:\frac{\mathrm{4000}}{\mathrm{28}}\:=\:\frac{\mathrm{1000}}{\mathrm{7}} \\ $$$$\mathrm{Moles}\:\mathrm{of}\:\mathrm{CO}_{\mathrm{2}} \:=\:\frac{\mathrm{6000}}{\mathrm{44}}\:=\:\frac{\mathrm{1500}}{\mathrm{11}} \\ $$$$\mathrm{Total}\:\mathrm{number}\:\mathrm{of}\:\mathrm{moles}\:=\:\frac{\mathrm{21500}}{\mathrm{77}} \\ $$$$\mathrm{Mole}\:\mathrm{fraction}\:\mathrm{of}\:\mathrm{N}_{\mathrm{2}} \:=\:\frac{\mathrm{1000}}{\mathrm{7}}×\frac{\mathrm{77}}{\mathrm{21500}} \\ $$$$\approx\:\mathrm{0}.\mathrm{512} \\ $$$$\mathrm{Mole}\:\mathrm{fraction}\:\mathrm{of}\:\mathrm{CO}_{\mathrm{2}} \:=\:\frac{\mathrm{1500}}{\mathrm{11}}×\frac{\mathrm{77}}{\mathrm{21500}} \\ $$$$\approx\:\mathrm{0}.\mathrm{488} \\ $$$$\mathrm{Average}\:\mathrm{molecular}\:\mathrm{weight} \\ $$$$=\:\mathrm{10000}×\frac{\mathrm{77}}{\mathrm{21500}}\:\approx\:\mathrm{35}.\mathrm{814} \\ $$$$\mathrm{Partial}\:\mathrm{pressure}\:\mathrm{of}\:\mathrm{N}_{\mathrm{2}} \:=\:\mathrm{0}.\mathrm{512}\:×\:\mathrm{4}\:\mathrm{bar} \\ $$$$=\:\mathrm{2}.\mathrm{048}\:\mathrm{bar} \\ $$$$\mathrm{Partial}\:\mathrm{pressure}\:\mathrm{of}\:\mathrm{CO}_{\mathrm{2}} \:=\:\mathrm{0}.\mathrm{488}\:×\:\mathrm{4}\:\mathrm{bar} \\ $$$$=\:\mathrm{1}.\mathrm{952}\:\mathrm{bar} \\ $$
Commented by tawa tawa last updated on 02/Jul/17
$$\mathrm{Wow},\:\mathrm{i}\:\mathrm{really}\:\mathrm{appreciate}\:\mathrm{sir}.\:\mathrm{God}\:\mathrm{bless}\:\mathrm{you}\:\mathrm{sir}. \\ $$
