Question Number 17209 by sushmitak last updated on 02/Jul/17
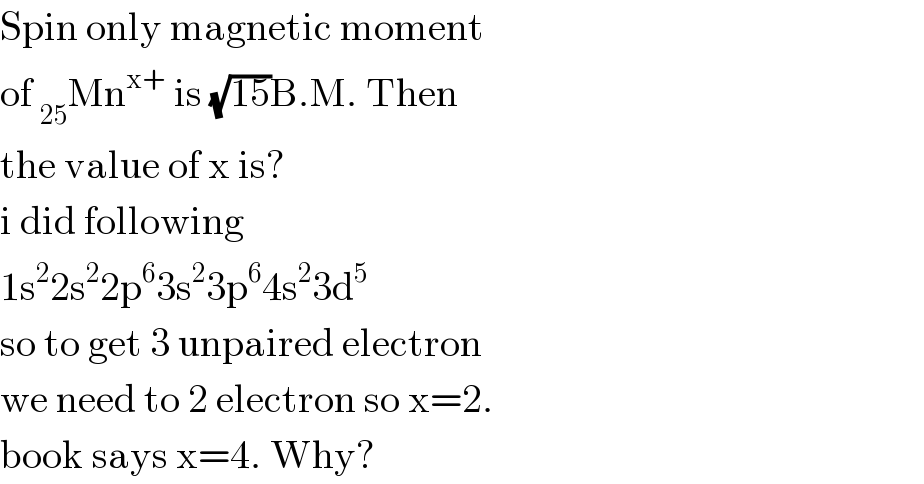
$$\mathrm{Spin}\:\mathrm{only}\:\mathrm{magnetic}\:\mathrm{moment} \\ $$$$\mathrm{of}\:_{\mathrm{25}} \mathrm{Mn}^{\mathrm{x}+} \:\mathrm{is}\:\sqrt{\mathrm{15}}\mathrm{B}.\mathrm{M}.\:\mathrm{Then} \\ $$$$\mathrm{the}\:\mathrm{value}\:\mathrm{of}\:\mathrm{x}\:\mathrm{is}? \\ $$$$\mathrm{i}\:\mathrm{did}\:\mathrm{following} \\ $$$$\mathrm{1s}^{\mathrm{2}} \mathrm{2s}^{\mathrm{2}} \mathrm{2p}^{\mathrm{6}} \mathrm{3s}^{\mathrm{2}} \mathrm{3p}^{\mathrm{6}} \mathrm{4s}^{\mathrm{2}} \mathrm{3d}^{\mathrm{5}} \\ $$$$\mathrm{so}\:\mathrm{to}\:\mathrm{get}\:\mathrm{3}\:\mathrm{unpaired}\:\mathrm{electron} \\ $$$$\mathrm{we}\:\mathrm{need}\:\mathrm{to}\:\mathrm{2}\:\mathrm{electron}\:\mathrm{so}\:\mathrm{x}=\mathrm{2}. \\ $$$$\mathrm{book}\:\mathrm{says}\:\mathrm{x}=\mathrm{4}.\:\mathrm{Why}? \\ $$
Answered by Tinkutara last updated on 02/Jul/17
![Book′s answer is correct. [Mn] = _(18) [Ar]4s^2 3d^5 and magnetic moment = (√(15)) BM So unpaired electrons = 3 Losing first 2 electrons from 4s orbital and next 2 from 3d orbital will result in _(18) [Ar]3d^3 which satisfies that number of unpaired electrons is 3. Hence it is Mn^(+4) .](https://www.tinkutara.com/question/Q17221.png)
$$\mathrm{Book}'\mathrm{s}\:\mathrm{answer}\:\mathrm{is}\:\mathrm{correct}. \\ $$$$\left[\mathrm{Mn}\right]\:=\:_{\mathrm{18}} \left[\mathrm{Ar}\right]\mathrm{4}{s}^{\mathrm{2}} \mathrm{3}{d}^{\mathrm{5}} \\ $$$$\mathrm{and}\:\mathrm{magnetic}\:\mathrm{moment}\:=\:\sqrt{\mathrm{15}}\:\mathrm{BM} \\ $$$$\mathrm{So}\:\mathrm{unpaired}\:\mathrm{electrons}\:=\:\mathrm{3} \\ $$$$\mathrm{Losing}\:\mathrm{first}\:\mathrm{2}\:\mathrm{electrons}\:\mathrm{from}\:\mathrm{4}{s}\:\mathrm{orbital} \\ $$$$\mathrm{and}\:\mathrm{next}\:\mathrm{2}\:\mathrm{from}\:\mathrm{3}{d}\:\mathrm{orbital}\:\mathrm{will}\:\mathrm{result} \\ $$$$\mathrm{in}\:_{\mathrm{18}} \left[\mathrm{Ar}\right]\mathrm{3}{d}^{\mathrm{3}} \:\mathrm{which}\:\mathrm{satisfies}\:\mathrm{that} \\ $$$$\mathrm{number}\:\mathrm{of}\:\mathrm{unpaired}\:\mathrm{electrons}\:\mathrm{is}\:\mathrm{3}. \\ $$$$\mathrm{Hence}\:\mathrm{it}\:\mathrm{is}\:\mathrm{Mn}^{+\mathrm{4}} . \\ $$
Commented by sushmitak last updated on 02/Jul/17

$$\mathrm{Thank}\:\mathrm{You}. \\ $$$$\mathrm{So}\:\mathrm{3d}\:\mathrm{fills}\:\mathrm{first}\:\mathrm{but}\:\mathrm{4s}\:\mathrm{is}\:\mathrm{first}\:\mathrm{to} \\ $$$$\mathrm{lose}\:\mathrm{during}\:\mathrm{ionization}? \\ $$
Commented by Tinkutara last updated on 02/Jul/17

$$\mathrm{Yes},\:\mathrm{exactly}. \\ $$
