Question Number 22050 by Tinkutara last updated on 10/Oct/17
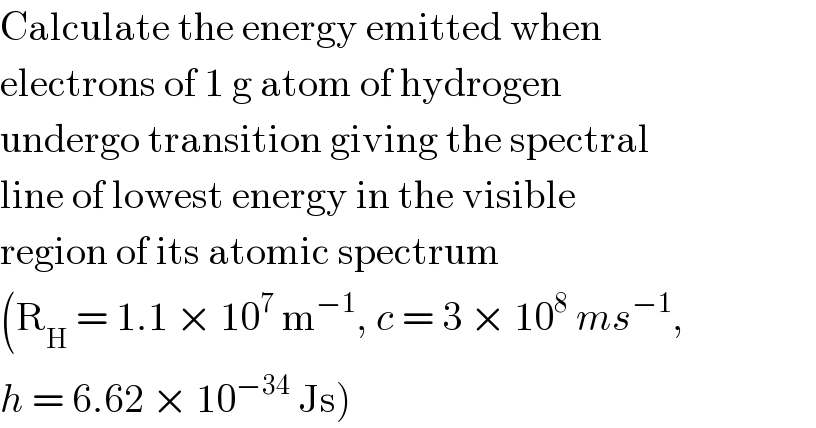
$$\mathrm{Calculate}\:\mathrm{the}\:\mathrm{energy}\:\mathrm{emitted}\:\mathrm{when} \\ $$$$\mathrm{electrons}\:\mathrm{of}\:\mathrm{1}\:\mathrm{g}\:\mathrm{atom}\:\mathrm{of}\:\mathrm{hydrogen} \\ $$$$\mathrm{undergo}\:\mathrm{transition}\:\mathrm{giving}\:\mathrm{the}\:\mathrm{spectral} \\ $$$$\mathrm{line}\:\mathrm{of}\:\mathrm{lowest}\:\mathrm{energy}\:\mathrm{in}\:\mathrm{the}\:\mathrm{visible} \\ $$$$\mathrm{region}\:\mathrm{of}\:\mathrm{its}\:\mathrm{atomic}\:\mathrm{spectrum} \\ $$$$\left(\mathrm{R}_{\mathrm{H}} \:=\:\mathrm{1}.\mathrm{1}\:×\:\mathrm{10}^{\mathrm{7}} \:\mathrm{m}^{−\mathrm{1}} ,\:{c}\:=\:\mathrm{3}\:×\:\mathrm{10}^{\mathrm{8}} \:{ms}^{−\mathrm{1}} ,\right. \\ $$$$\left.{h}\:=\:\mathrm{6}.\mathrm{62}\:×\:\mathrm{10}^{−\mathrm{34}} \:\mathrm{Js}\right) \\ $$
