Question Number 22883 by Tinkutara last updated on 23/Oct/17
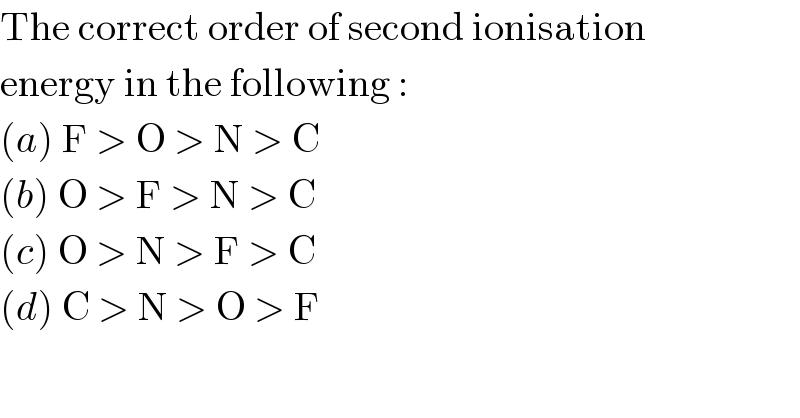
$$\mathrm{The}\:\mathrm{correct}\:\mathrm{order}\:\mathrm{of}\:\mathrm{second}\:\mathrm{ionisation} \\ $$$$\mathrm{energy}\:\mathrm{in}\:\mathrm{the}\:\mathrm{following}\:: \\ $$$$\left({a}\right)\:\mathrm{F}\:>\:\mathrm{O}\:>\:\mathrm{N}\:>\:\mathrm{C} \\ $$$$\left({b}\right)\:\mathrm{O}\:>\:\mathrm{F}\:>\:\mathrm{N}\:>\:\mathrm{C} \\ $$$$\left({c}\right)\:\mathrm{O}\:>\:\mathrm{N}\:>\:\mathrm{F}\:>\:\mathrm{C} \\ $$$$\left({d}\right)\:\mathrm{C}\:>\:\mathrm{N}\:>\:\mathrm{O}\:>\:\mathrm{F} \\ $$
Commented by math solver last updated on 23/Oct/17

$${B}\:? \\ $$
Answered by math solver last updated on 23/Oct/17

$${draw}\:{electronic}\:{configuration}\:{of}\:{each}… \\ $$$$\mathrm{2}{nd}\:{I}.{E}\:{means}\:{removing}\:{electron} \\ $$$${from}\:{monovalent}\:{cation}…. \\ $$$$\mathrm{2}{p}^{\mathrm{3}\:\:} \:\rangle\:\mathrm{2}{p}^{\mathrm{4}} \:\left\{\:{in}\:{case}\:{of}\:{stability}\:\right\} \\ $$$${becaouse}\:\mathrm{2}\:{p}^{\mathrm{3}} \:{is}\:{half}\:{filled}\:{and}\:{the} \\ $$$${rest}\:{you}\:{know}….. \\ $$
Commented by Tinkutara last updated on 24/Oct/17
$$\mathrm{Thank}\:\mathrm{you}\:\mathrm{very}\:\mathrm{much}\:\mathrm{Sir}! \\ $$
