Question Number 22999 by Tinkutara last updated on 24/Oct/17
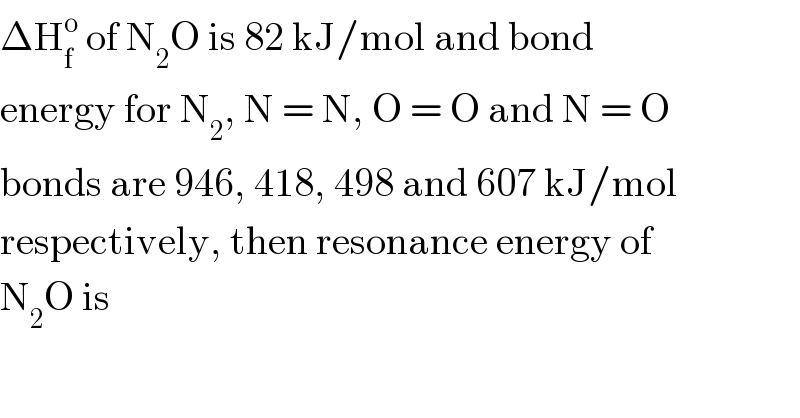
$$\Delta\mathrm{H}_{\mathrm{f}} ^{\mathrm{o}} \:\mathrm{of}\:\mathrm{N}_{\mathrm{2}} \mathrm{O}\:\mathrm{is}\:\mathrm{82}\:\mathrm{kJ}/\mathrm{mol}\:\mathrm{and}\:\mathrm{bond} \\ $$$$\mathrm{energy}\:\mathrm{for}\:\mathrm{N}_{\mathrm{2}} ,\:\mathrm{N}\:=\:\mathrm{N},\:\mathrm{O}\:=\:\mathrm{O}\:\mathrm{and}\:\mathrm{N}\:=\:\mathrm{O} \\ $$$$\mathrm{bonds}\:\mathrm{are}\:\mathrm{946},\:\mathrm{418},\:\mathrm{498}\:\mathrm{and}\:\mathrm{607}\:\mathrm{kJ}/\mathrm{mol} \\ $$$$\mathrm{respectively},\:\mathrm{then}\:\mathrm{resonance}\:\mathrm{energy}\:\mathrm{of} \\ $$$$\mathrm{N}_{\mathrm{2}} \mathrm{O}\:\mathrm{is} \\ $$
