Question Number 23715 by Tinkutara last updated on 04/Nov/17
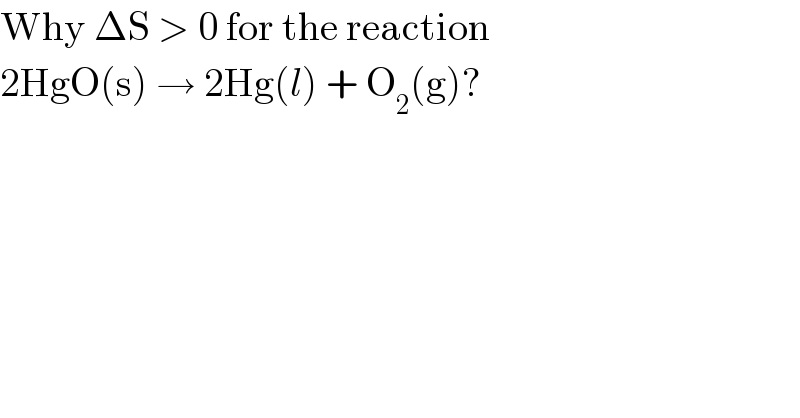
$$\mathrm{Why}\:\Delta\mathrm{S}\:>\:\mathrm{0}\:\mathrm{for}\:\mathrm{the}\:\mathrm{reaction} \\ $$$$\mathrm{2HgO}\left(\mathrm{s}\right)\:\rightarrow\:\mathrm{2Hg}\left({l}\right)\:+\:\mathrm{O}_{\mathrm{2}} \left(\mathrm{g}\right)? \\ $$
