Question Number 23981 by Tinkutara last updated on 10/Nov/17

$$\mathrm{Amount}\:\mathrm{of}\:\mathrm{heat}\:\mathrm{required}\:\mathrm{to}\:\mathrm{change}\:\mathrm{1}\:{g} \\ $$$$\mathrm{ice}\:\mathrm{at}\:\mathrm{0}°\mathrm{C}\:\mathrm{to}\:\mathrm{1}\:{g}\:\mathrm{steam}\:\mathrm{at}\:\mathrm{100}°\mathrm{C}\:\mathrm{is} \\ $$$$\left(\mathrm{1}\right)\:\mathrm{616}\:\mathrm{cal} \\ $$$$\left(\mathrm{2}\right)\:\mathrm{12}\:\mathrm{kcal} \\ $$$$\left(\mathrm{3}\right)\:\mathrm{717}\:\mathrm{cal} \\ $$$$\left(\mathrm{4}\right)\:\mathrm{none}\:\mathrm{of}\:\mathrm{these}. \\ $$
Answered by ajfour last updated on 10/Nov/17
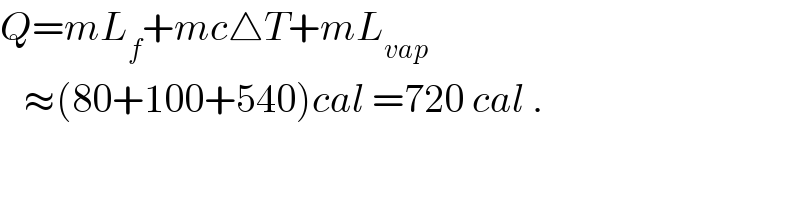
$${Q}={mL}_{{f}} +{mc}\bigtriangleup{T}+{mL}_{{vap}} \\ $$$$\:\:\:\approx\left(\mathrm{80}+\mathrm{100}+\mathrm{540}\right){cal}\:=\mathrm{720}\:{cal}\:. \\ $$
Commented by Tinkutara last updated on 10/Nov/17
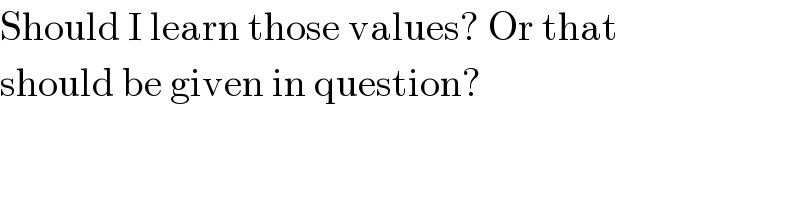
$$\mathrm{Should}\:\mathrm{I}\:\mathrm{learn}\:\mathrm{those}\:\mathrm{values}?\:\mathrm{Or}\:\mathrm{that} \\ $$$$\mathrm{should}\:\mathrm{be}\:\mathrm{given}\:\mathrm{in}\:\mathrm{question}? \\ $$
Commented by ajfour last updated on 10/Nov/17
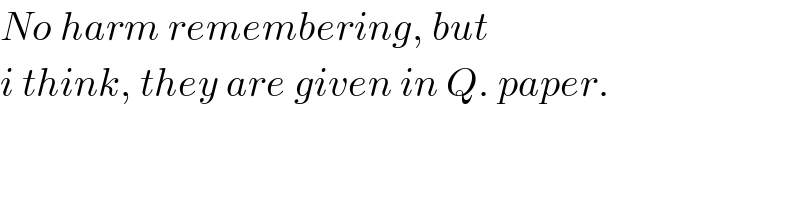
$${No}\:{harm}\:{remembering},\:{but} \\ $$$${i}\:{think},\:{they}\:{are}\:{given}\:{in}\:{Q}.\:{paper}. \\ $$
Commented by Tinkutara last updated on 10/Nov/17

$$\mathrm{OK}\:\mathrm{Thanks}. \\ $$
