Question Number 31209 by rahul@ last updated on 03/Mar/18
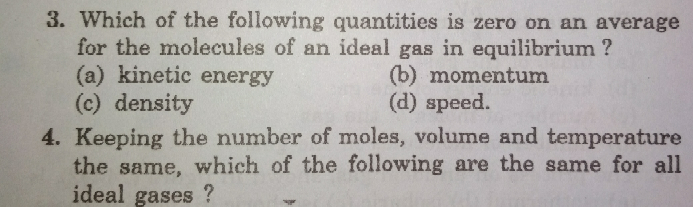
Commented by rahul@ last updated on 03/Mar/18
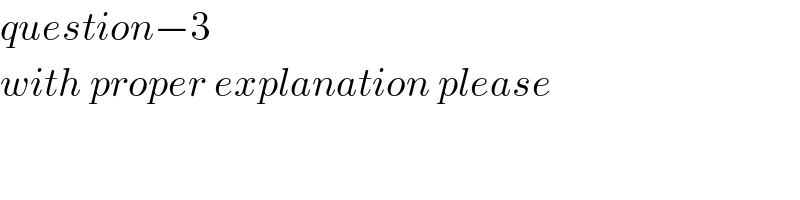
$${question}−\mathrm{3} \\ $$$${with}\:{proper}\:{explanation}\:{please} \\ $$
Commented by ajfour last updated on 03/Mar/18
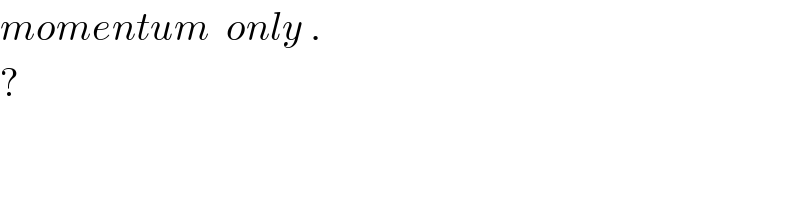
$${momentum}\:\:{only}\:.\:\:\: \\ $$$$? \\ $$
Answered by tanmay.chaudhury50@gmail.com last updated on 20/May/18
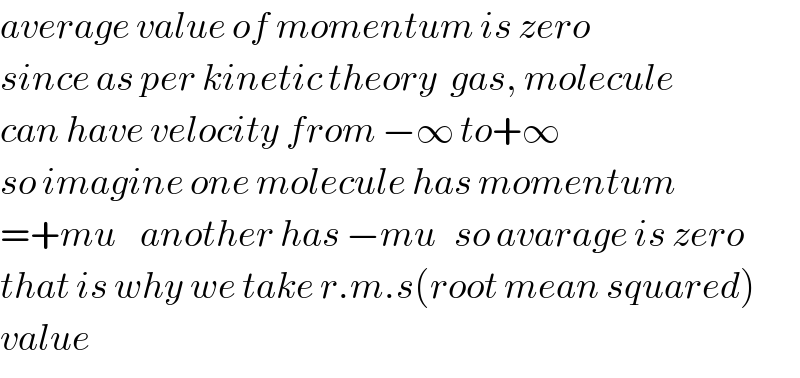
$${average}\:{value}\:{of}\:{momentum}\:{is}\:{zero} \\ $$$${since}\:{as}\:{per}\:{kinetic}\:{theory}\:\:{gas},\:{molecule} \\ $$$${can}\:{have}\:{velocity}\:{from}\:−\infty\:{to}+\infty \\ $$$${so}\:{imagine}\:{one}\:{molecule}\:{has}\:{momentum} \\ $$$$=+{mu}\:\:\:\:{another}\:{has}\:−{mu}\:\:\:{so}\:{avarage}\:{is}\:{zero} \\ $$$${that}\:{is}\:{why}\:{we}\:{take}\:{r}.{m}.{s}\left({root}\:{mean}\:{squared}\right) \\ $$$${value} \\ $$
