Question Number 31906 by Tinkutara last updated on 16/Mar/18

Answered by rahul 19 last updated on 18/Mar/18
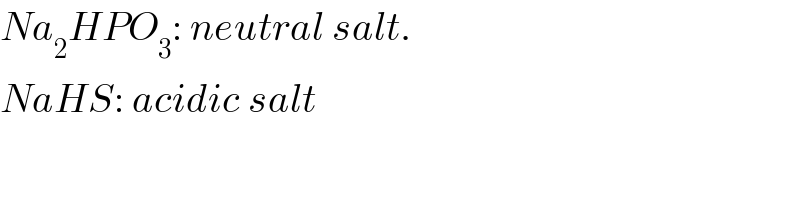
$${Na}_{\mathrm{2}} {HPO}_{\mathrm{3}} :\:{neutral}\:{salt}. \\ $$$${NaHS}:\:{acidic}\:{salt} \\ $$
Commented by Tinkutara last updated on 19/Mar/18
Why NaHS=acidic salt? Na is strong base and H2S is weak acid so why not basic salt?
Commented by rahul 19 last updated on 19/Mar/18
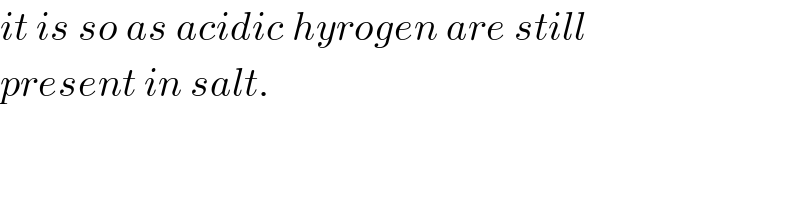
$${it}\:{is}\:{so}\:{as}\:{acidic}\:{hyrogen}\:{are}\:{still} \\ $$$${present}\:{in}\:{salt}. \\ $$
Commented by Tinkutara last updated on 19/Mar/18
But acidic salt means its pH<7 but due to strong base Na it's pH>7. Isn't it wrong then?
Commented by rahul 19 last updated on 19/Mar/18

$${intermidiates}\:{formed}\:{during}\:{the} \\ $$$${reaction}\:{of}\:\boldsymbol{{polyprotic}}\:{acids}\:{with} \\ $$$${base}\:{are}\:{acidic}\:.\:{and}\:{the}\:{final}\:{product} \\ $$$${is}\:{basic}\:\left({Na}_{\mathrm{2}} {S}\right).\:{this}\:{is}\:{also}\:{applicable} \\ $$$${for}\:\boldsymbol{{polyprotic}}\:{bases}! \\ $$
Commented by Tinkutara last updated on 19/Mar/18
Thank you very much Sir! I got the answer. ��������
