Question Number 46558 by azharkhan250963@gmail.com last updated on 28/Oct/18

$${calculate}\:{the}\:{uncertainty}\:{in}\:{velocity}\:{of}\:{an}\:{electron}\:{which}\:{is}\:{confined}\:{in}\:{a}\:\mathrm{10}^{−\mathrm{10}\:} {meter} \\ $$
Answered by tanmay.chaudhury50@gmail.com last updated on 28/Oct/18
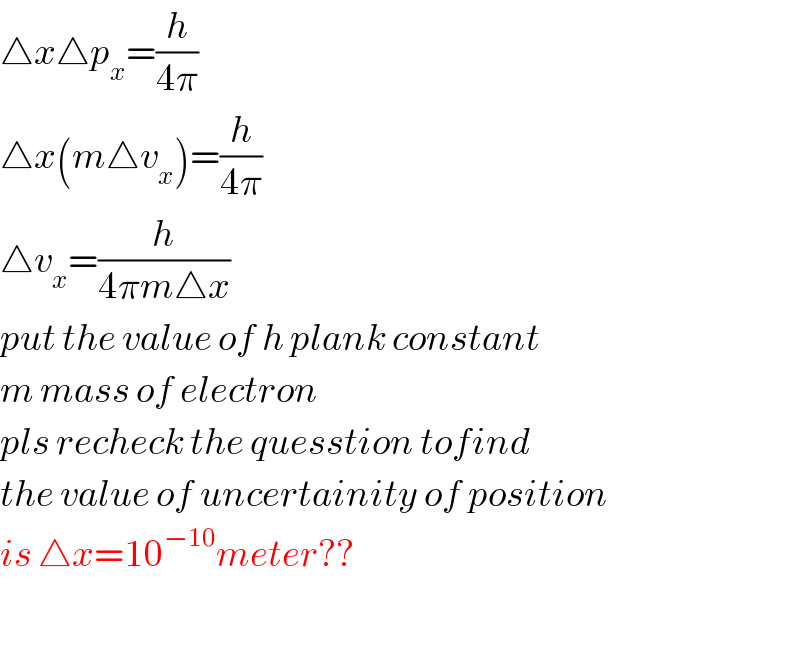
$$\bigtriangleup{x}\bigtriangleup{p}_{{x}} =\frac{{h}}{\mathrm{4}\pi} \\ $$$$\bigtriangleup{x}\left({m}\bigtriangleup{v}_{{x}} \right)=\frac{{h}}{\mathrm{4}\pi} \\ $$$$\bigtriangleup{v}_{{x}} =\frac{{h}}{\mathrm{4}\pi{m}\bigtriangleup{x}} \\ $$$${put}\:{the}\:{value}\:{of}\:{h}\:{plank}\:{constant} \\ $$$${m}\:{mass}\:{of}\:{electron} \\ $$$${pls}\:{recheck}\:{the}\:{quesstion}\:{tofind}\: \\ $$$${the}\:{value}\:{of}\:{uncertainity}\:{of}\:{position} \\ $$$${is}\:\bigtriangleup{x}=\mathrm{10}^{−\mathrm{10}} {meter}?? \\ $$$$ \\ $$
