Question Number 131935 by Salman_Abir last updated on 09/Feb/21
Answered by physicstutes last updated on 10/Feb/21
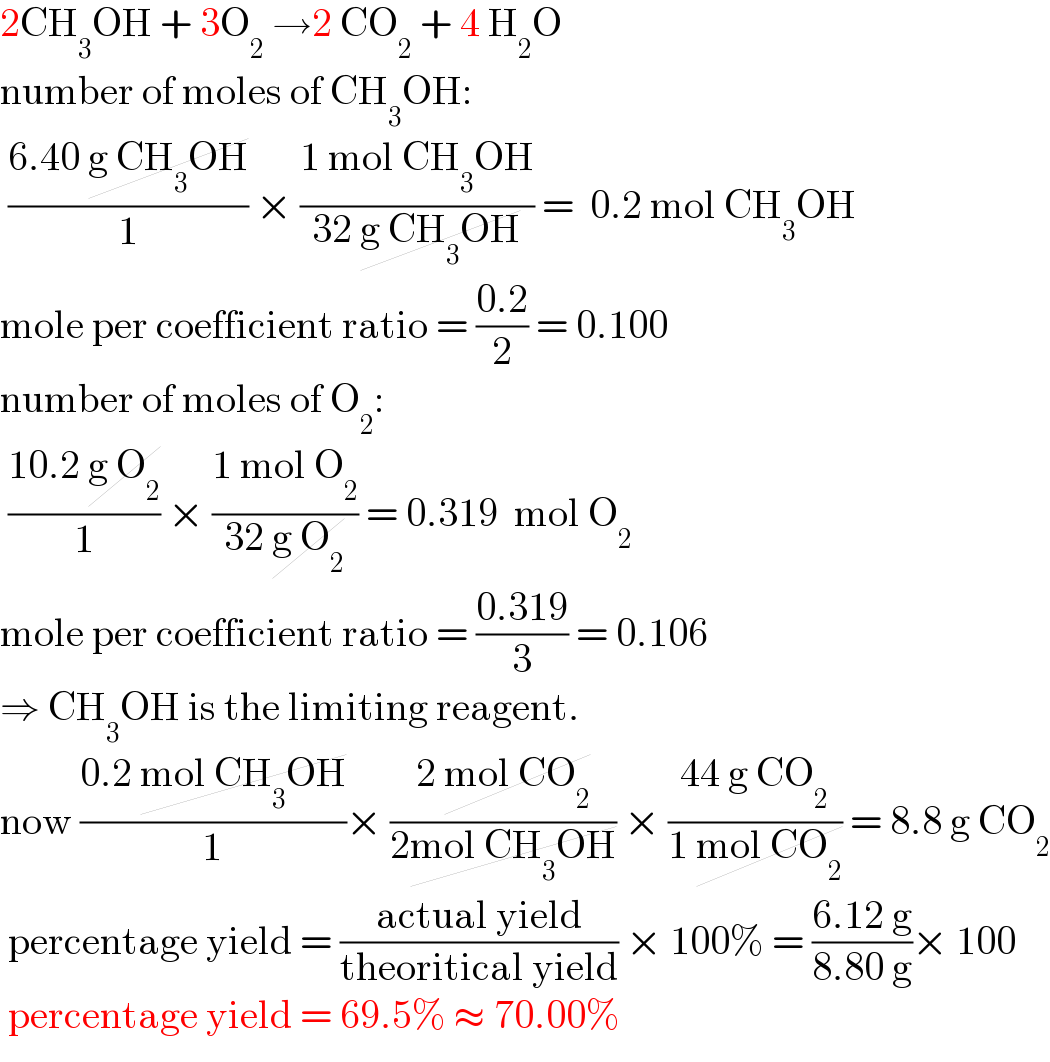
$$\mathrm{2CH}_{\mathrm{3}} \mathrm{OH}\:+\:\mathrm{3O}_{\mathrm{2}} \:\rightarrow\mathrm{2}\:\mathrm{CO}_{\mathrm{2}} \:+\:\mathrm{4}\:\mathrm{H}_{\mathrm{2}} \mathrm{O} \\ $$$$\mathrm{number}\:\mathrm{of}\:\mathrm{moles}\:\mathrm{of}\:\mathrm{CH}_{\mathrm{3}} \mathrm{OH}: \\ $$$$\:\frac{\mathrm{6}.\mathrm{40}\:\cancel{\mathrm{g}\:\mathrm{CH}_{\mathrm{3}} \mathrm{OH}}}{\mathrm{1}}\:×\:\frac{\mathrm{1}\:\mathrm{mol}\:\mathrm{CH}_{\mathrm{3}} \mathrm{OH}}{\mathrm{32}\:\cancel{\mathrm{g}\:\mathrm{CH}_{\mathrm{3}} \mathrm{OH}}}\:=\:\:\mathrm{0}.\mathrm{2}\:\mathrm{mol}\:\mathrm{CH}_{\mathrm{3}} \mathrm{OH} \\ $$$$\mathrm{mole}\:\mathrm{per}\:\mathrm{coefficient}\:\mathrm{ratio}\:=\:\frac{\mathrm{0}.\mathrm{2}}{\mathrm{2}}\:=\:\mathrm{0}.\mathrm{100} \\ $$$$\mathrm{number}\:\mathrm{of}\:\mathrm{moles}\:\mathrm{of}\:\mathrm{O}_{\mathrm{2}} : \\ $$$$\:\frac{\mathrm{10}.\mathrm{2}\:\cancel{\mathrm{g}\:\mathrm{O}_{\mathrm{2}} }}{\mathrm{1}}\:×\:\frac{\mathrm{1}\:\mathrm{mol}\:\mathrm{O}_{\mathrm{2}} }{\mathrm{32}\:\cancel{\mathrm{g}\:\mathrm{O}_{\mathrm{2}} }}\:=\:\mathrm{0}.\mathrm{319}\:\:\mathrm{mol}\:\mathrm{O}_{\mathrm{2}} \\ $$$$\mathrm{mole}\:\mathrm{per}\:\mathrm{coefficient}\:\mathrm{ratio}\:=\:\frac{\mathrm{0}.\mathrm{319}}{\mathrm{3}}\:=\:\mathrm{0}.\mathrm{106}\: \\ $$$$\Rightarrow\:\mathrm{CH}_{\mathrm{3}} \mathrm{OH}\:\mathrm{is}\:\mathrm{the}\:\mathrm{limiting}\:\mathrm{reagent}. \\ $$$$\mathrm{now}\:\frac{\mathrm{0}.\mathrm{2}\:\cancel{\mathrm{mol}\:\mathrm{CH}_{\mathrm{3}} \mathrm{OH}}}{\mathrm{1}}×\:\frac{\mathrm{2}\:\cancel{\mathrm{mol}\:\mathrm{CO}_{\mathrm{2}} }}{\mathrm{2}\cancel{\mathrm{mol}\:\mathrm{CH}_{\mathrm{3}} \mathrm{OH}}}\:×\:\frac{\mathrm{44}\:\mathrm{g}\:\mathrm{CO}_{\mathrm{2}} }{\mathrm{1}\:\cancel{\mathrm{mol}\:\mathrm{CO}_{\mathrm{2}} }}\:=\:\mathrm{8}.\mathrm{8}\:\mathrm{g}\:\mathrm{CO}_{\mathrm{2}} \\ $$$$\:\mathrm{percentage}\:\mathrm{yield}\:=\:\frac{\mathrm{actual}\:\mathrm{yield}}{\mathrm{theoritical}\:\mathrm{yield}}\:×\:\mathrm{100\%}\:=\:\frac{\mathrm{6}.\mathrm{12}\:\mathrm{g}}{\mathrm{8}.\mathrm{80}\:\mathrm{g}}×\:\mathrm{100}\: \\ $$$$\:\mathrm{percentage}\:\mathrm{yield}\:=\:\mathrm{69}.\mathrm{5\%}\:\approx\:\mathrm{70}.\mathrm{00\%} \\ $$
