Question Number 11389 by tawa last updated on 23/Mar/17
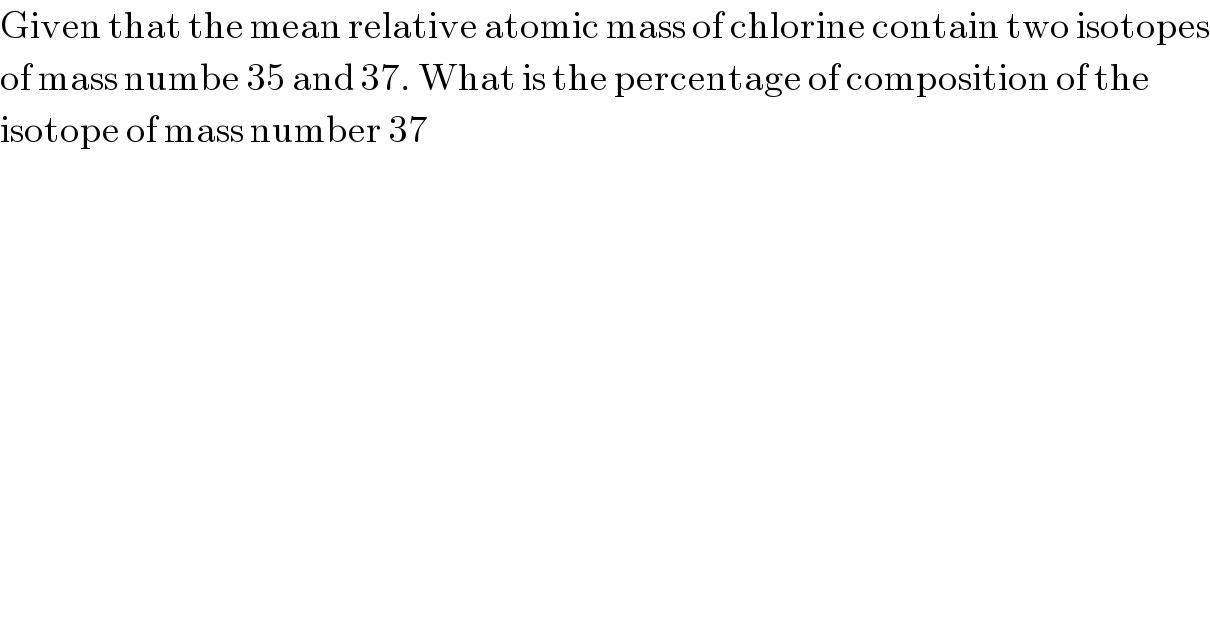
$$\mathrm{Given}\:\mathrm{that}\:\mathrm{the}\:\mathrm{mean}\:\mathrm{relative}\:\mathrm{atomic}\:\mathrm{mass}\:\mathrm{of}\:\mathrm{chlorine}\:\mathrm{contain}\:\mathrm{two}\:\mathrm{isotopes} \\ $$$$\mathrm{of}\:\mathrm{mass}\:\mathrm{numbe}\:\mathrm{35}\:\mathrm{and}\:\mathrm{37}.\:\mathrm{What}\:\mathrm{is}\:\mathrm{the}\:\mathrm{percentage}\:\mathrm{of}\:\mathrm{composition}\:\mathrm{of}\:\mathrm{the} \\ $$$$\mathrm{isotope}\:\mathrm{of}\:\mathrm{mass}\:\mathrm{number}\:\mathrm{37}\: \\ $$
Answered by sandy_suhendra last updated on 23/Mar/17

$$\mathrm{isotope}\:\mathrm{of}\:\mathrm{mass}\:\mathrm{number}\:\mathrm{37}=\mathrm{x\%} \\ $$$$\mathrm{isotope}\:\mathrm{of}\:\mathrm{mass}\:\mathrm{number}\:\mathrm{36}=\left(\mathrm{100}−\mathrm{x}\right)\% \\ $$$$\mathrm{the}\:\mathrm{mean}\:\mathrm{relative}\:\mathrm{atomic}\:\mathrm{mass}\:\mathrm{of}\:\mathrm{Cl}=\mathrm{35}.\mathrm{5}\:\:\: \\ $$$$\mathrm{35}.\mathrm{5}=\frac{\mathrm{x}}{\mathrm{100}}×\mathrm{37}\:+\:\frac{\mathrm{100}−\mathrm{x}}{\mathrm{100}}×\mathrm{35} \\ $$$$\mathrm{3550}=\mathrm{37x}+\mathrm{3500}−\mathrm{35x} \\ $$$$\mathrm{50}=\mathrm{2x} \\ $$$$\mathrm{x}=\mathrm{25\%} \\ $$
Commented by tawa last updated on 23/Mar/17

$$\mathrm{God}\:\mathrm{bless}\:\mathrm{you}\:\mathrm{sir}. \\ $$
