Question Number 12801 by tawa last updated on 01/May/17

Answered by sandy_suhendra last updated on 01/May/17
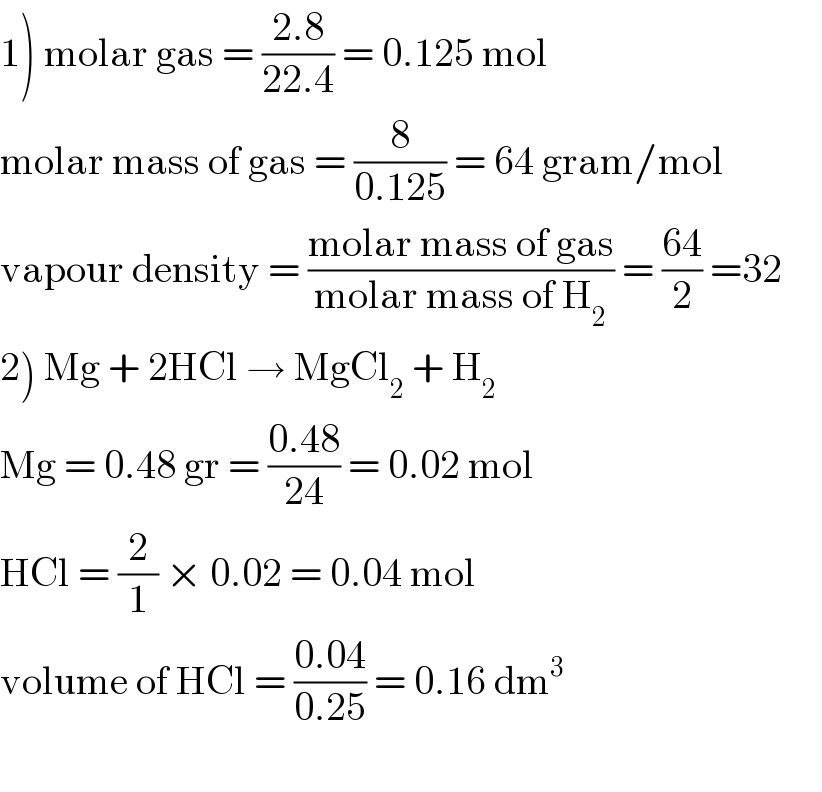
$$\left.\mathrm{1}\right)\:\mathrm{molar}\:\mathrm{gas}\:=\:\frac{\mathrm{2}.\mathrm{8}}{\mathrm{22}.\mathrm{4}}\:=\:\mathrm{0}.\mathrm{125}\:\mathrm{mol} \\ $$$$\mathrm{molar}\:\mathrm{mass}\:\mathrm{of}\:\mathrm{gas}\:=\:\frac{\mathrm{8}}{\mathrm{0}.\mathrm{125}}\:=\:\mathrm{64}\:\mathrm{gram}/\mathrm{mol} \\ $$$$\mathrm{vapour}\:\mathrm{density}\:=\:\frac{\mathrm{molar}\:\mathrm{mass}\:\mathrm{of}\:\mathrm{gas}}{\mathrm{molar}\:\mathrm{mass}\:\mathrm{of}\:\mathrm{H}_{\mathrm{2}} }\:=\:\frac{\mathrm{64}}{\mathrm{2}}\:=\mathrm{32}\:\:\:\:\: \\ $$$$\left.\mathrm{2}\right)\:\mathrm{Mg}\:+\:\mathrm{2HCl}\:\rightarrow\:\mathrm{MgCl}_{\mathrm{2}} \:+\:\mathrm{H}_{\mathrm{2}} \\ $$$$\mathrm{Mg}\:=\:\mathrm{0}.\mathrm{48}\:\mathrm{gr}\:=\:\frac{\mathrm{0}.\mathrm{48}}{\mathrm{24}}\:=\:\mathrm{0}.\mathrm{02}\:\mathrm{mol} \\ $$$$\mathrm{HCl}\:=\:\frac{\mathrm{2}}{\mathrm{1}}\:×\:\mathrm{0}.\mathrm{02}\:=\:\mathrm{0}.\mathrm{04}\:\mathrm{mol} \\ $$$$\mathrm{volume}\:\mathrm{of}\:\mathrm{HCl}\:=\:\frac{\mathrm{0}.\mathrm{04}}{\mathrm{0}.\mathrm{25}}\:=\:\mathrm{0}.\mathrm{16}\:\mathrm{dm}^{\mathrm{3}} \\ $$$$ \\ $$
Commented by tawa last updated on 01/May/17

$$\mathrm{God}\:\mathrm{bless}\:\mathrm{you}\:\mathrm{sir} \\ $$
