
Question and Answers Forum
Question Number 10023 by Tawakalitu ayo mi last updated on 21/Jan/17

Answered by ridwan balatif last updated on 21/Jan/17
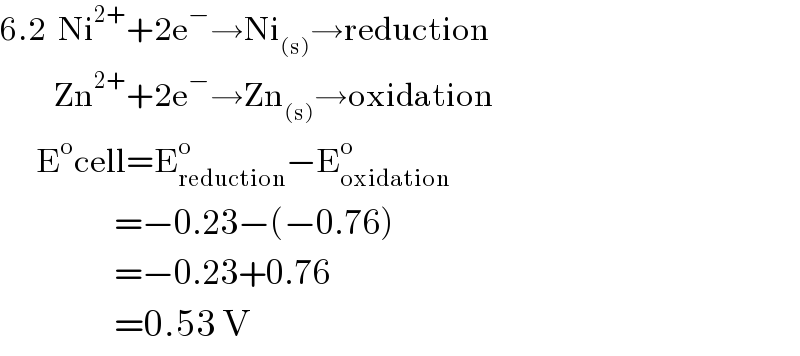
Commented by Tawakalitu ayo mi last updated on 21/Jan/17

Answered by ridwan balatif last updated on 21/Jan/17

Commented by Tawakalitu ayo mi last updated on 21/Jan/17

