
Question Number 10214 by Tawakalitu ayo mi last updated on 30/Jan/17
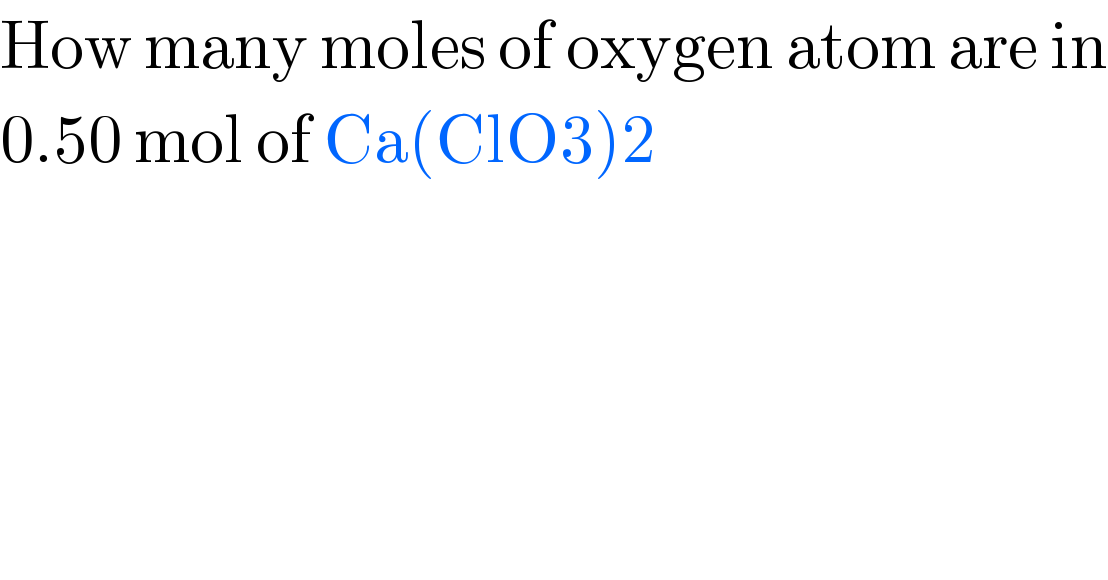
$$\mathrm{How}\:\mathrm{many}\:\mathrm{moles}\:\mathrm{of}\:\mathrm{oxygen}\:\mathrm{atom}\:\mathrm{are}\:\mathrm{in} \\ $$$$\mathrm{0}.\mathrm{50}\:\mathrm{mol}\:\mathrm{of}\:\mathrm{Ca}\left(\mathrm{ClO3}\right)\mathrm{2} \\ $$
Commented by sandy_suhendra last updated on 31/Jan/17
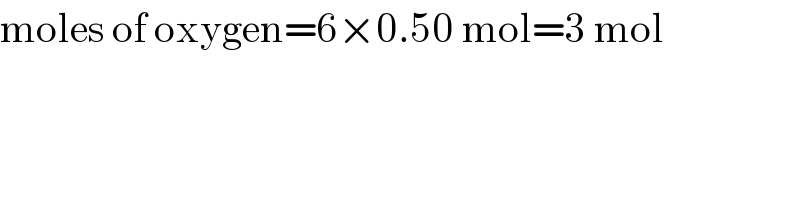
$$\mathrm{moles}\:\mathrm{of}\:\mathrm{oxygen}=\mathrm{6}×\mathrm{0}.\mathrm{50}\:\mathrm{mol}=\mathrm{3}\:\mathrm{mol} \\ $$
Answered by ridwan balatif last updated on 30/Jan/17

$$\mathrm{Mr}\:\mathrm{Ca}\left(\mathrm{ClO}_{\mathrm{3}} \right)_{\mathrm{2}} =\mathrm{Ar}.\mathrm{Ca}+\mathrm{2}×\mathrm{ArCl}+\mathrm{6}×\mathrm{Ar}.\mathrm{O}=\mathrm{40}+\mathrm{2}×\mathrm{35}.\mathrm{5}+\mathrm{6}×\mathrm{12}=\mathrm{183}\:\mathrm{g}/\mathrm{mole} \\ $$$$\mathrm{mole}\:\mathrm{of}\:\mathrm{oxygen}\:\mathrm{atom}=\frac{\mathrm{6}×\mathrm{Ar}.\mathrm{oxygen}}{\mathrm{Mr}.\mathrm{compound}}×\mathrm{mole}.\mathrm{compound} \\ $$$$\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:=\frac{\mathrm{72}}{\mathrm{183}}×\mathrm{0}.\mathrm{5}\approx\mathrm{0}.\mathrm{196mole} \\ $$
Commented by Tawakalitu ayo mi last updated on 30/Jan/17
$$\mathrm{God}\:\mathrm{bless}\:\mathrm{you}\:\mathrm{sir}. \\ $$
