
Question Number 150761 by DELETED last updated on 15/Aug/21

Answered by DELETED last updated on 15/Aug/21
![1.a). (NH_4 )_2 Cr_2 O_7 →N_2 +4H_2 O+Cr_2 O_3 2[N^(−3) H_4 ^(+1) ]^+ + [Cr_2 ^(+6) O_7 ^(−2) ]^(−2) →N_2 ^0 +4H_2 ^(+1) O^(−2) +C^(+3) r_2 O_3 ^(−2) no 1 a disebut reaksi redoks , karena bilangan oksida atom ada yg berubah antara sebelum dan sesudah reaksi.](Q150763.png)
$$\left.\mathrm{1}.\mathrm{a}\right).\:\left(\mathrm{NH}_{\mathrm{4}} \right)_{\mathrm{2}} \mathrm{Cr}_{\mathrm{2}} \mathrm{O}_{\mathrm{7}} \rightarrow\mathrm{N}_{\mathrm{2}} +\mathrm{4H}_{\mathrm{2}} \mathrm{O}+\mathrm{Cr}_{\mathrm{2}} \mathrm{O}_{\mathrm{3}} \\ $$$$\:\:\:\mathrm{2}\left[\overset{−\mathrm{3}} {\mathrm{N}}\overset{+\mathrm{1}} {\mathrm{H}}_{\mathrm{4}} \overset{+} {\right]}+\:\left[\mathrm{C}\overset{+\mathrm{6}} {\mathrm{r}}_{\mathrm{2}} \overset{−\mathrm{2}} {\mathrm{O}}_{\mathrm{7}} \right]^{−\mathrm{2}} \:\rightarrow\overset{\mathrm{0}} {\mathrm{N}}_{\mathrm{2}} \:+\mathrm{4}\overset{+\mathrm{1}} {\mathrm{H}}_{\mathrm{2}} \overset{−\mathrm{2}} {\mathrm{O}}+\overset{+\mathrm{3}} {\mathrm{C}r}_{\mathrm{2}} \overset{−\mathrm{2}} {\mathrm{O}}_{\mathrm{3}} \\ $$$$\:\:\:\mathrm{no}\:\mathrm{1}\:\mathrm{a}\:\mathrm{disebut}\:\mathrm{reaksi}\:\mathrm{redoks}\:,\:\mathrm{karena} \\ $$$$\:\:\mathrm{bilangan}\:\mathrm{oksida}\:\mathrm{atom}\:\mathrm{ada}\:\mathrm{yg} \\ $$$$\:\:\mathrm{berubah}\:\mathrm{antara}\:\mathrm{sebelum}\:\mathrm{dan}\:\mathrm{sesudah} \\ $$$$\:\:\mathrm{reaksi}. \\ $$$$\:\:\:\:\:\:\: \\ $$
Answered by DELETED last updated on 15/Aug/21

$$\left.\mathrm{1}\:\mathrm{b}\right).\:\mathrm{CuCO}_{\mathrm{3}} +\mathrm{H}_{\mathrm{2}} \mathrm{SO}_{\mathrm{4}} \rightarrow\mathrm{CuSO}_{\mathrm{4}} +\mathrm{H}_{\mathrm{2}} \mathrm{O}+\mathrm{CO}_{\mathrm{2}} \\ $$$$\:\:\:\:\:\:\:\:\:\:\:\mathrm{C}\overset{+\mathrm{2}} {\mathrm{u}}\mid\left(\overset{+\mathrm{4}} {\mathrm{C}}\overset{−\mathrm{2}} {\mathrm{O}}_{\mathrm{3}} \right)^{−\mathrm{2}} +\overset{+\mathrm{1}} {\mathrm{H}}_{\mathrm{2}} \mid\left(\overset{+\mathrm{6}} {\mathrm{S}}\overset{−\mathrm{2}} {\mathrm{O}}_{\mathrm{4}} \right)^{−\mathrm{2}} \rightarrow\mathrm{C}\overset{+\mathrm{2}} {\mathrm{u}}\mid\left(\overset{+\mathrm{6}} {\mathrm{S}}\overset{−\mathrm{2}} {\mathrm{O}}_{\mathrm{4}} \right)^{−\mathrm{2}} +\overset{+\mathrm{1}} {\mathrm{H}}_{\mathrm{2}} \mid\overset{−\mathrm{3}} {\mathrm{O}}+\overset{+\mathrm{4}} {\mathrm{C}}\mid\overset{−\mathrm{2}} {\mathrm{O}}_{\mathrm{2}} \\ $$$$\:\:\:\:\:\mathrm{bukan}\:\mathrm{reaksi}\:\mathrm{redoks}\:\mathrm{karena}\:\mathrm{biloks} \\ $$$$\:\:\:\:\:\:\mathrm{tdk}\:\mathrm{ada}\:\mathrm{yg}\:\mathrm{berubah} \\ $$$$ \\ $$
Answered by DELETED last updated on 15/Aug/21

$$\left.\mathrm{1c}\right).\:\mathrm{H}_{\mathrm{2}} \mathrm{S}+\mathrm{2H}_{\mathrm{2}} \mathrm{O}+\mathrm{3Cl}_{\mathrm{2}} \rightarrow\mathrm{SO}_{\mathrm{2}} +\mathrm{6HCl} \\ $$$$\:\:\:\:\:\:\:\:\:\:\overset{+\mathrm{1}} {\mathrm{H}}_{\mathrm{2}} \overset{−\mathrm{2}} {\mathrm{S}}+\mathrm{2}\overset{+\mathrm{1}} {\mathrm{H}}_{\mathrm{2}} \overset{−\mathrm{2}} {\mathrm{O}}+\mathrm{3}\overset{\mathrm{0}} {\mathrm{C}l}_{\mathrm{2}} \rightarrow\overset{+\mathrm{4}} {\mathrm{S}}\overset{−\mathrm{2}} {\mathrm{O}}_{\mathrm{2}} +\mathrm{6}\overset{+\mathrm{1}} {\mathrm{H}}\overset{−\mathrm{1}} {\mathrm{C}l} \\ $$$$\:\:\:\:\:\:\:\:\mathrm{termasuk}\:\mathrm{reaksi}\:\mathrm{redoks}.\:\mathrm{karena} \\ $$$$\:\:\:\:\:\:\:\:\:\:\mathrm{biloksnya}\:\mathrm{berubah}. \\ $$$$ \\ $$
Answered by DELETED last updated on 15/Aug/21
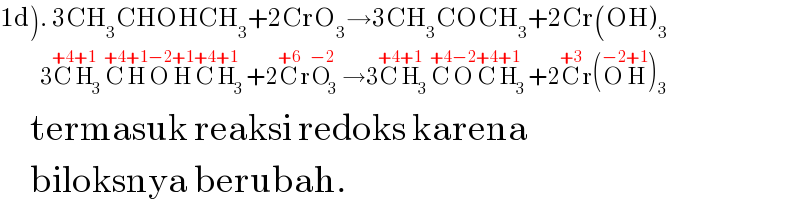
$$\left.\mathrm{1d}\right).\:\mathrm{3CH}_{\mathrm{3}} \mathrm{CHOHCH}_{\mathrm{3}} +\mathrm{2CrO}_{\mathrm{3}} \rightarrow\mathrm{3CH}_{\mathrm{3}} \mathrm{COCH}_{\mathrm{3}} +\mathrm{2Cr}\left(\mathrm{OH}\right)_{\mathrm{3}} \\ $$$$\:\:\:\:\:\:\:\:\:\:\mathrm{3}\overset{+\mathrm{4}} {\mathrm{C}}\overset{+\mathrm{1}} {\mathrm{H}}_{\mathrm{3}} \overset{+\mathrm{4}} {\mathrm{C}}\overset{+\mathrm{1}} {\mathrm{H}}\overset{−\mathrm{2}} {\mathrm{O}}\overset{+\mathrm{1}} {\mathrm{H}}\overset{+\mathrm{4}} {\mathrm{C}}\overset{+\mathrm{1}} {\mathrm{H}}_{\mathrm{3}} +\mathrm{2}\overset{+\mathrm{6}} {\mathrm{C}r}\overset{−\mathrm{2}} {\mathrm{O}}_{\mathrm{3}} \rightarrow\mathrm{3}\overset{+\mathrm{4}} {\mathrm{C}}\overset{+\mathrm{1}} {\mathrm{H}}_{\mathrm{3}} \overset{+\mathrm{4}} {\mathrm{C}}\overset{−\mathrm{2}} {\mathrm{O}}\overset{+\mathrm{4}} {\mathrm{C}}\overset{+\mathrm{1}} {\mathrm{H}}_{\mathrm{3}} +\mathrm{2}\overset{+\mathrm{3}} {\mathrm{C}r}\left(\overset{−\mathrm{2}} {\mathrm{O}}\overset{+\mathrm{1}} {\mathrm{H}}\right)_{\mathrm{3}} \\ $$$$\:\:\:\:\:\mathrm{termasuk}\:\mathrm{reaksi}\:\mathrm{redoks}\:\mathrm{karena} \\ $$$$\:\:\:\:\:\mathrm{biloksnya}\:\mathrm{berubah}. \\ $$
Answered by DELETED last updated on 15/Aug/21
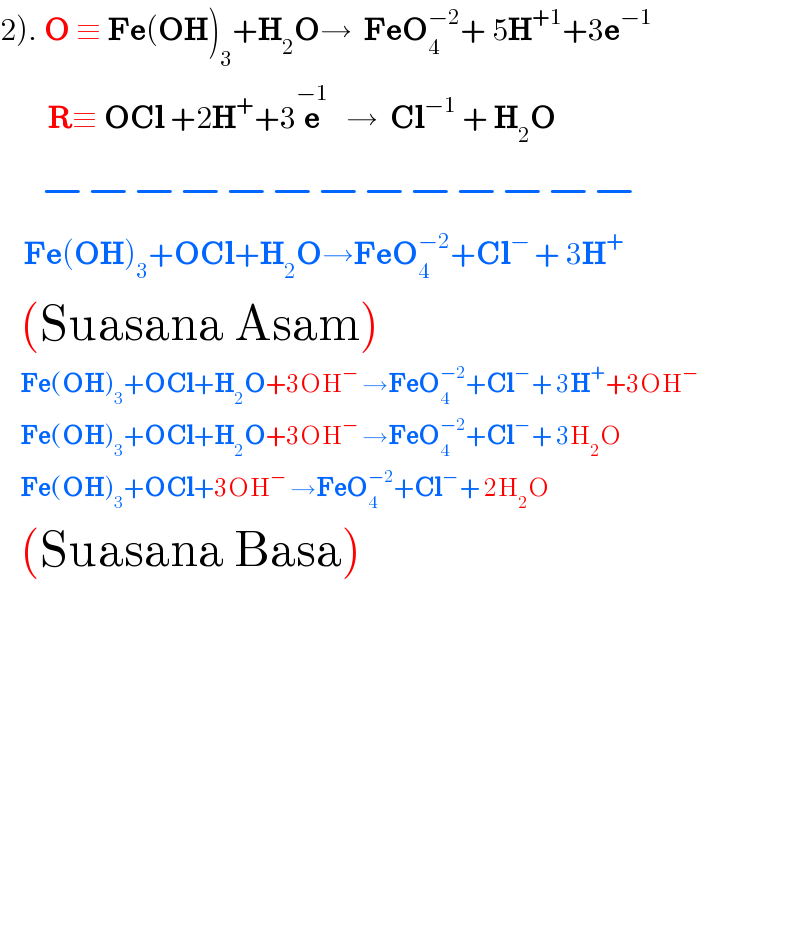
$$\left.\mathrm{2}\right).\:\boldsymbol{\mathrm{O}}\:\equiv\:\boldsymbol{\mathrm{Fe}}\left(\boldsymbol{\mathrm{OH}}\right)_{\mathrm{3}} +\boldsymbol{\mathrm{H}}_{\mathrm{2}} \boldsymbol{\mathrm{O}}\rightarrow\:\:\boldsymbol{\mathrm{FeO}}_{\mathrm{4}} ^{−\mathrm{2}} +\:\mathrm{5}\boldsymbol{\mathrm{H}}^{+\mathrm{1}} +\mathrm{3}\boldsymbol{\mathrm{e}}^{−\mathrm{1}} \\ $$$$\:\:\:\:\:\:\:\:\boldsymbol{\mathrm{R}}\equiv\:\boldsymbol{\mathrm{OCl}}\:+\mathrm{2}\boldsymbol{\mathrm{H}}^{+} +\mathrm{3}\overset{−\mathrm{1}} {\boldsymbol{\mathrm{e}}}\:\:\:\rightarrow\:\:\boldsymbol{\mathrm{Cl}}^{−\mathrm{1}} \:+\:\boldsymbol{\mathrm{H}}_{\mathrm{2}} \boldsymbol{\mathrm{O}}\: \\ $$$$\:\:\:\:−−−−−−−−−−−−− \\ $$$$\:\:\:\:\boldsymbol{\mathrm{Fe}}\left(\boldsymbol{\mathrm{OH}}\right)_{\mathrm{3}} +\boldsymbol{\mathrm{OCl}}+\boldsymbol{\mathrm{H}}_{\mathrm{2}} \boldsymbol{\mathrm{O}}\rightarrow\boldsymbol{\mathrm{FeO}}_{\mathrm{4}} ^{−\mathrm{2}} +\boldsymbol{\mathrm{Cl}}^{−\:} +\:\mathrm{3}\boldsymbol{\mathrm{H}}^{+} \\ $$$$\:\:\left(\mathrm{Suasana}\:\mathrm{Asam}\right) \\ $$$$\:\:\:\:\:\boldsymbol{\mathrm{Fe}}\left(\boldsymbol{\mathrm{OH}}\right)_{\mathrm{3}} +\boldsymbol{\mathrm{OCl}}+\boldsymbol{\mathrm{H}}_{\mathrm{2}} \boldsymbol{\mathrm{O}}+\mathrm{3OH}^{−} \:\rightarrow\boldsymbol{\mathrm{FeO}}_{\mathrm{4}} ^{−\mathrm{2}} +\boldsymbol{\mathrm{Cl}}^{−\:} +\:\mathrm{3}\boldsymbol{\mathrm{H}}^{+} +\mathrm{3OH}^{−} \\ $$$$\:\:\:\:\:\boldsymbol{\mathrm{Fe}}\left(\boldsymbol{\mathrm{OH}}\right)_{\mathrm{3}} +\boldsymbol{\mathrm{OCl}}+\boldsymbol{\mathrm{H}}_{\mathrm{2}} \boldsymbol{\mathrm{O}}+\mathrm{3OH}^{−} \:\rightarrow\boldsymbol{\mathrm{FeO}}_{\mathrm{4}} ^{−\mathrm{2}} +\boldsymbol{\mathrm{Cl}}^{−\:} +\:\mathrm{3H}_{\mathrm{2}} \mathrm{O} \\ $$$$\:\:\:\:\:\boldsymbol{\mathrm{Fe}}\left(\boldsymbol{\mathrm{OH}}\right)_{\mathrm{3}} +\boldsymbol{\mathrm{OCl}}+\mathrm{3OH}^{−} \:\rightarrow\boldsymbol{\mathrm{FeO}}_{\mathrm{4}} ^{−\mathrm{2}} +\boldsymbol{\mathrm{Cl}}^{−\:} +\:\mathrm{2H}_{\mathrm{2}} \mathrm{O} \\ $$$$\:\:\left(\mathrm{Suasana}\:\mathrm{Basa}\right) \\ $$$$\: \\ $$$$ \\ $$$$\:\:\: \\ $$$$\:\:\: \\ $$$$\:\:\: \\ $$
Answered by DELETED last updated on 15/Aug/21
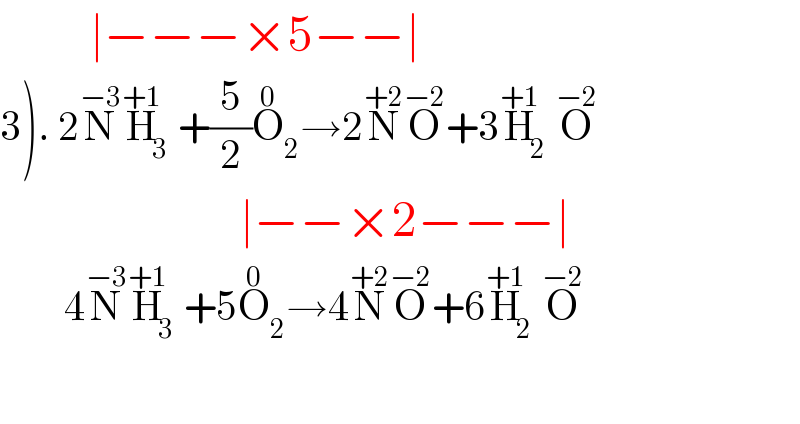
$$\:\:\:\:\:\:\:\:\:\mid−−−×\mathrm{5}−−\mid \\ $$$$\left.\mathrm{3}\right).\:\mathrm{2}\overset{−\mathrm{3}} {\mathrm{N}}\overset{+\mathrm{1}} {\mathrm{H}}_{\mathrm{3}} +\frac{\mathrm{5}}{\mathrm{2}}\overset{\mathrm{0}} {\mathrm{O}}_{\mathrm{2}} \rightarrow\mathrm{2}\overset{+\mathrm{2}} {\mathrm{N}}\overset{−\mathrm{2}} {\mathrm{O}}+\mathrm{3}\overset{+\mathrm{1}} {\mathrm{H}}_{\mathrm{2}} \overset{−\mathrm{2}} {\mathrm{O}} \\ $$$$\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\:\mid−−×\mathrm{2}−−−\mid \\ $$$$\:\:\:\:\:\:\:\:\mathrm{4}\overset{−\mathrm{3}} {\mathrm{N}}\overset{+\mathrm{1}} {\mathrm{H}}_{\mathrm{3}} +\mathrm{5}\overset{\mathrm{0}} {\mathrm{O}}_{\mathrm{2}} \rightarrow\mathrm{4}\overset{+\mathrm{2}} {\mathrm{N}}\overset{−\mathrm{2}} {\mathrm{O}}+\mathrm{6}\overset{+\mathrm{1}} {\mathrm{H}}_{\mathrm{2}} \overset{−\mathrm{2}} {\mathrm{O}} \\ $$$$\:\:\:\:\:\:\:\: \\ $$
