
Question and Answers Forum
Question Number 17209 by sushmitak last updated on 02/Jul/17
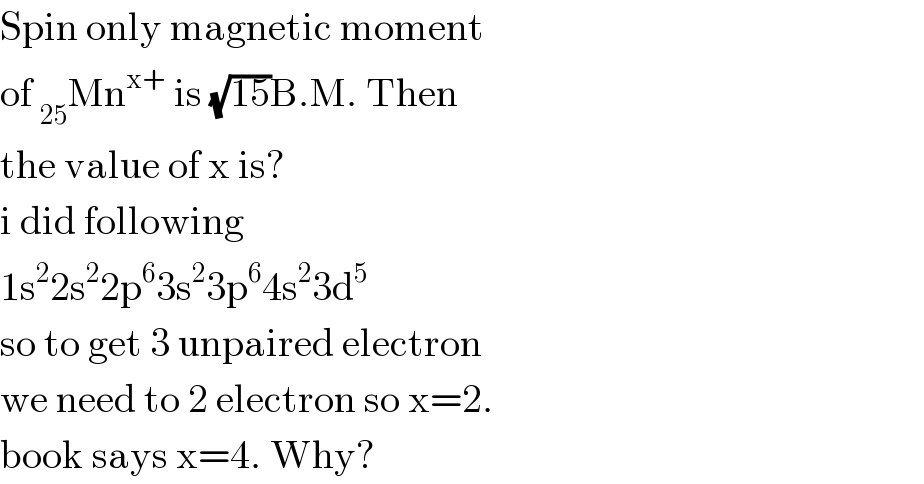
Answered by Tinkutara last updated on 02/Jul/17
![Book′s answer is correct. [Mn] = _(18) [Ar]4s^2 3d^5 and magnetic moment = (√(15)) BM So unpaired electrons = 3 Losing first 2 electrons from 4s orbital and next 2 from 3d orbital will result in _(18) [Ar]3d^3 which satisfies that number of unpaired electrons is 3. Hence it is Mn^(+4) .](Q17221.png)
Commented by sushmitak last updated on 02/Jul/17

Commented by Tinkutara last updated on 02/Jul/17

| ||
Question and Answers Forum | ||
Question Number 17209 by sushmitak last updated on 02/Jul/17 | ||
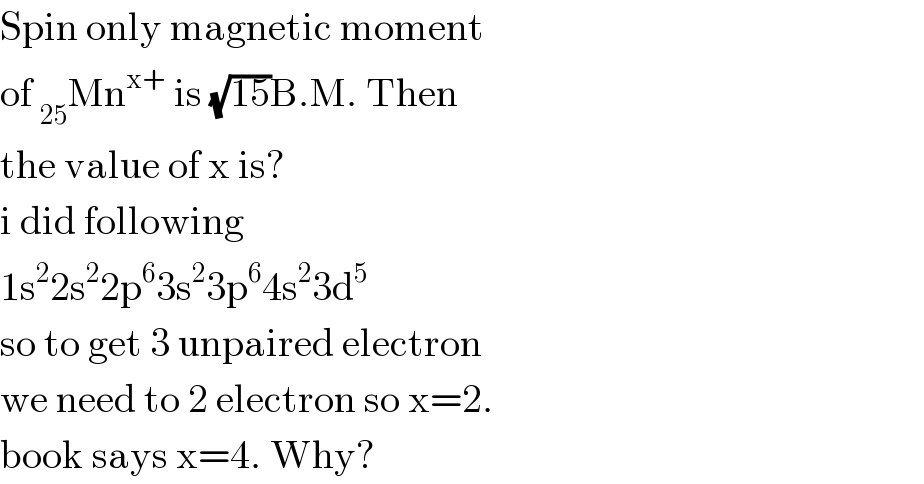 | ||
Answered by Tinkutara last updated on 02/Jul/17 | ||
![Book′s answer is correct. [Mn] = _(18) [Ar]4s^2 3d^5 and magnetic moment = (√(15)) BM So unpaired electrons = 3 Losing first 2 electrons from 4s orbital and next 2 from 3d orbital will result in _(18) [Ar]3d^3 which satisfies that number of unpaired electrons is 3. Hence it is Mn^(+4) .](Q17221.png) | ||
| ||
Commented by sushmitak last updated on 02/Jul/17 | ||
 | ||
Commented by Tinkutara last updated on 02/Jul/17 | ||
 | ||