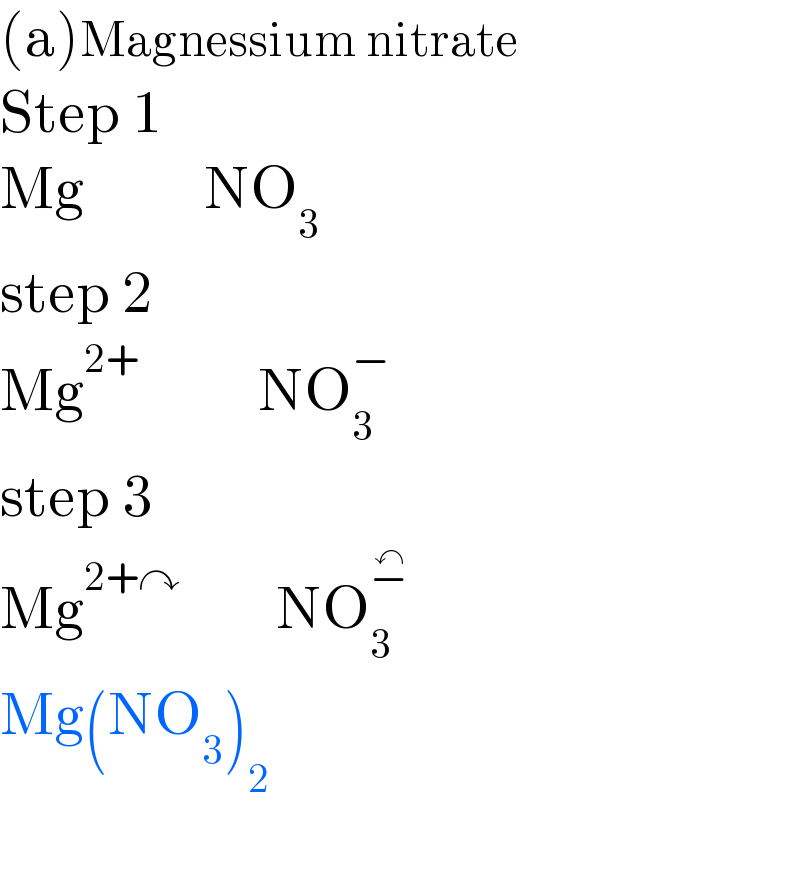Question and Answers Forum
Question Number 177742 by Spillover last updated on 08/Oct/22

Answered by a.lgnaoui last updated on 08/Oct/22
![(a)Magnesium Nitrate or (Nitate de magnesium) Mg(NO_3 )_2 [ Mg^(2+) 2NO_3 ^− ] (b) Aluminium Sulfate (Sulfate d aluninium) AL_2 (SO_4 )_3 [ 2AL^(3+) 3SO_4 ^(2−) ]](Q177749.png)
Answered by Tawa11 last updated on 08/Oct/22
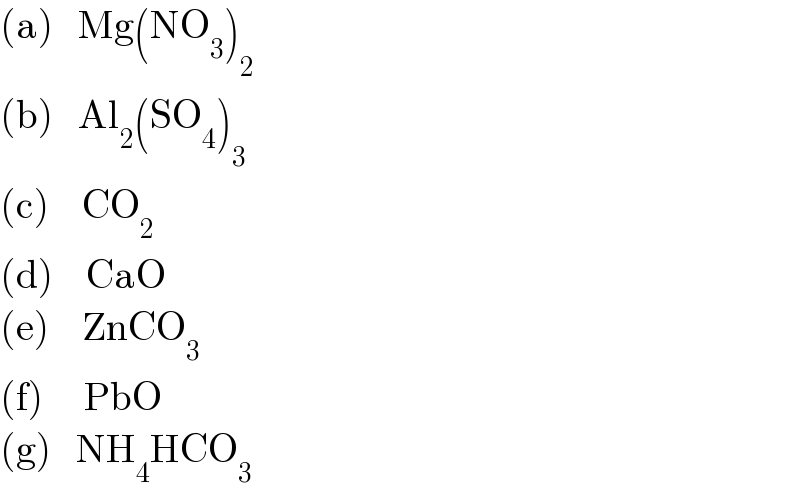
Commented by Spillover last updated on 08/Oct/22

Answered by Spillover last updated on 09/Oct/22