
Question and Answers Forum
Question Number 178009 by Spillover last updated on 12/Oct/22

Commented by Beginner last updated on 12/Oct/22

Commented by Spillover last updated on 12/Oct/22
![yes your right.thank you for reminding me.[C=12 O=16]](Q178017.png)
Answered by a.lgnaoui last updated on 12/Oct/22
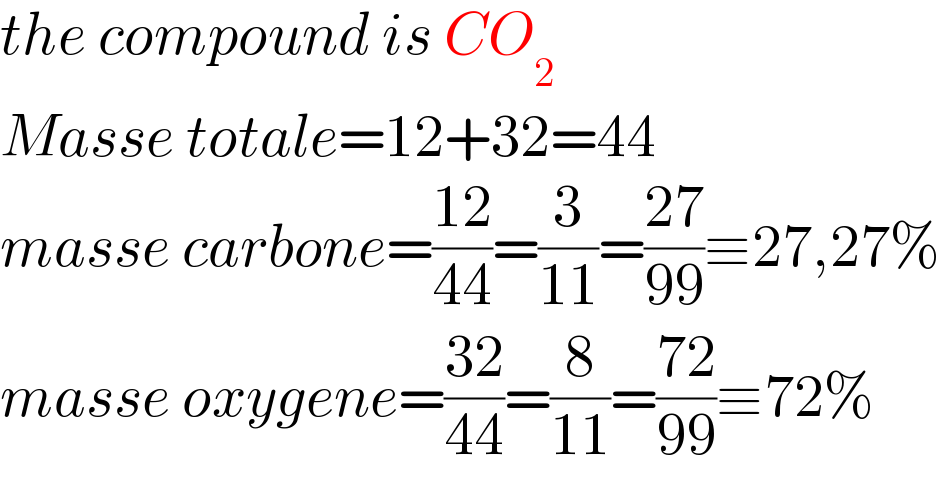
Commented by Spillover last updated on 12/Oct/22

Answered by Spillover last updated on 12/Oct/22

Answered by Spillover last updated on 12/Oct/22

