Question and Answers Forum
Question Number 178011 by Spillover last updated on 12/Oct/22
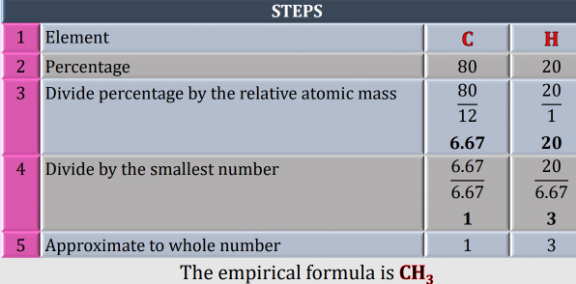
![Hydrocarbon contain 80% by mass of carbon and the rest is for hydrogen.calculate the empirical formula of the compound [given H=1 C=12]](Q178011.png)
Answered by a.lgnaoui last updated on 12/Oct/22
![the composant C_x H_y masse carbine(M_c = 12x →y(fir hudrigene) masse titaleM=12x+y Mc=(4/5)M=(4/5)(12x+y) wuth C_n H_(2n) (alcane)[x=2y] m(csebine=12→4fir hydrogene(exclu−:m(carbone=3×masse hydrigene) so the composant is: Alcene C_n H_(2n+2) (with n>1) n=2 C_2 H_6 (masse carbone =24→6for hydrogene) masse carbone 24=4×masse hydrogene) masse totale=30 → (((24)/(30))=(4/5) )=80% M(satisfait) so the composant is C_2 H_6](Q178056.png)
Commented by Spillover last updated on 12/Oct/22

Answered by Spillover last updated on 12/Oct/22
Answered by Spillover last updated on 12/Oct/22