
Question and Answers Forum
Question Number 178165 by Spillover last updated on 13/Oct/22

Answered by a.lgnaoui last updated on 14/Oct/22

Commented by Spillover last updated on 14/Oct/22

Answered by Spillover last updated on 14/Oct/22
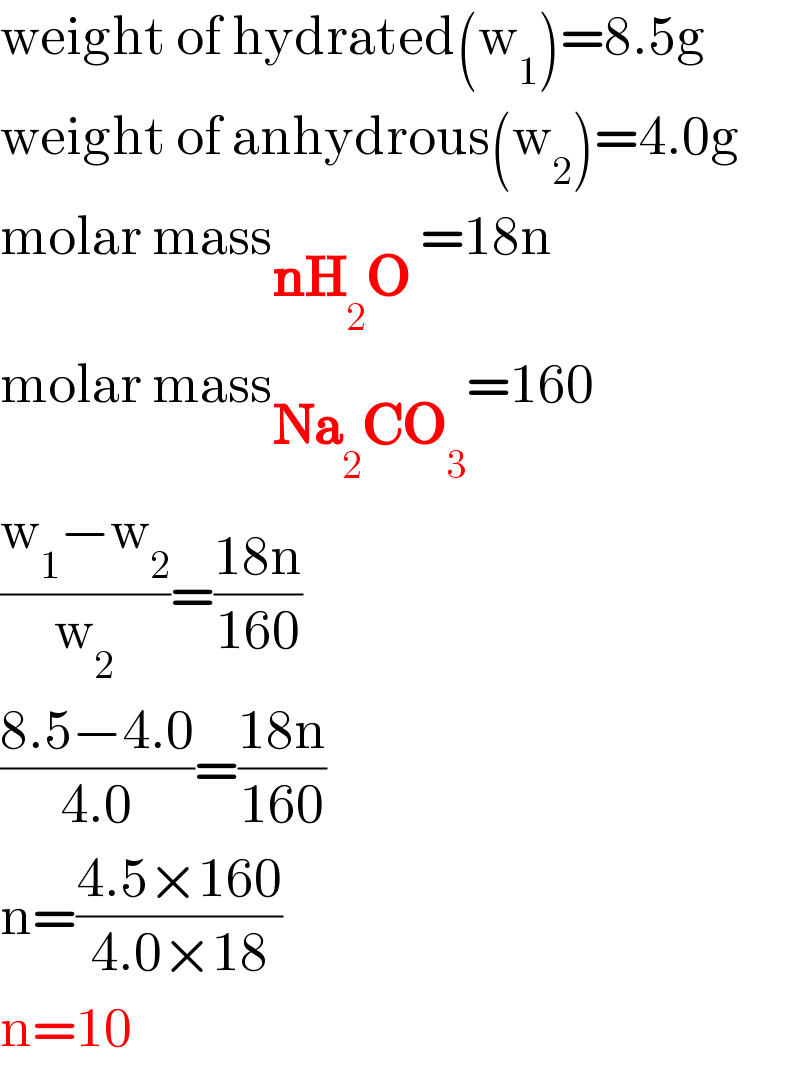
| ||
Question and Answers Forum | ||
Question Number 178165 by Spillover last updated on 13/Oct/22 | ||
 | ||
Answered by a.lgnaoui last updated on 14/Oct/22 | ||
 | ||
| ||
Commented by Spillover last updated on 14/Oct/22 | ||
 | ||
Answered by Spillover last updated on 14/Oct/22 | ||
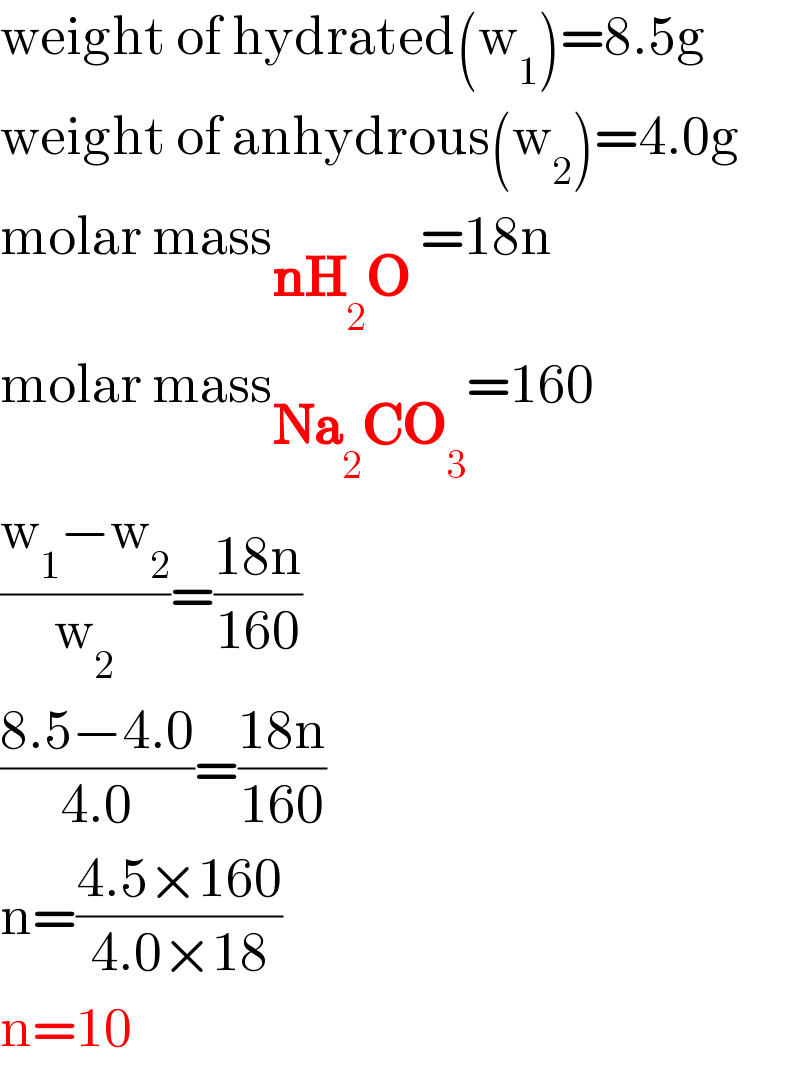 | ||
| ||