
Question and Answers Forum
Question Number 178166 by Spillover last updated on 13/Oct/22
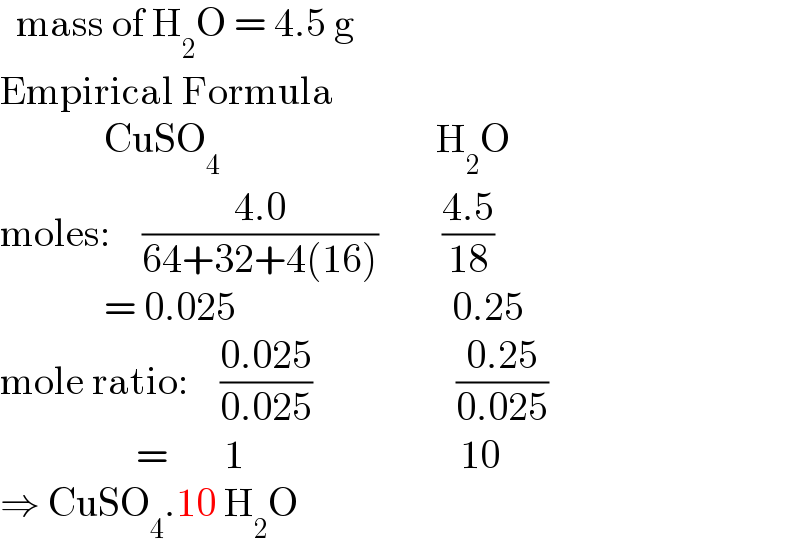
![8.5g of hydrated copper (ii) sulphate CuSO_4 .xH_2 O was heated to dryness.if 4.0g of anhydrous copper (ii) sulphate[CuSO_4 ] obtain Find number of molecule of water of crystallization](Q178166.png)
Answered by MikeH last updated on 14/Oct/22
Commented by Spillover last updated on 16/Oct/22

| ||
Question and Answers Forum | ||
Question Number 178166 by Spillover last updated on 13/Oct/22 | ||
![8.5g of hydrated copper (ii) sulphate CuSO_4 .xH_2 O was heated to dryness.if 4.0g of anhydrous copper (ii) sulphate[CuSO_4 ] obtain Find number of molecule of water of crystallization](Q178166.png) | ||
Answered by MikeH last updated on 14/Oct/22 | ||
 | ||
| ||
Commented by Spillover last updated on 16/Oct/22 | ||
 | ||