
Question and Answers Forum
Question Number 180902 by nadovic last updated on 19/Nov/22
![Calculate the root mean square speed of the molecules of a Helium gas kept in a gas cylinder at 400K. [Take R = 8.3 Jmol^(−1) K^(−1) ] The answer provided is 1.58 kms^(−1) Please I need help with the solution](Q180902.png)
Answered by Sam09 last updated on 19/Nov/22
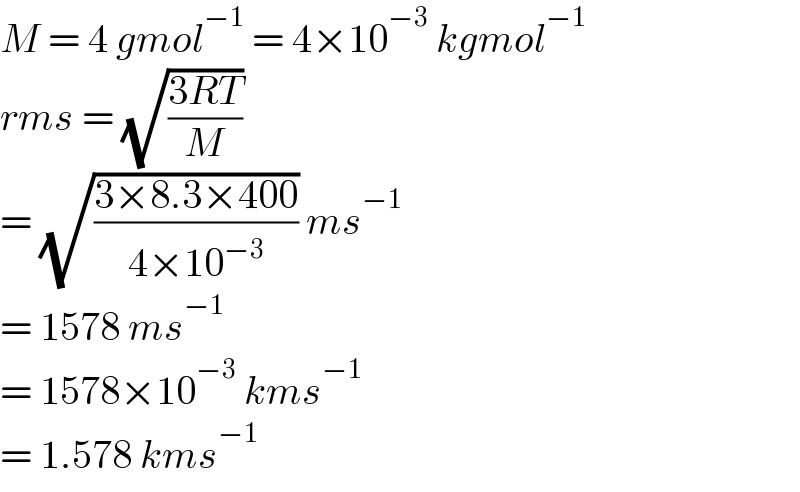
Commented by nadovic last updated on 11/Dec/22

| ||
Question and Answers Forum | ||
Question Number 180902 by nadovic last updated on 19/Nov/22 | ||
![Calculate the root mean square speed of the molecules of a Helium gas kept in a gas cylinder at 400K. [Take R = 8.3 Jmol^(−1) K^(−1) ] The answer provided is 1.58 kms^(−1) Please I need help with the solution](Q180902.png) | ||
Answered by Sam09 last updated on 19/Nov/22 | ||
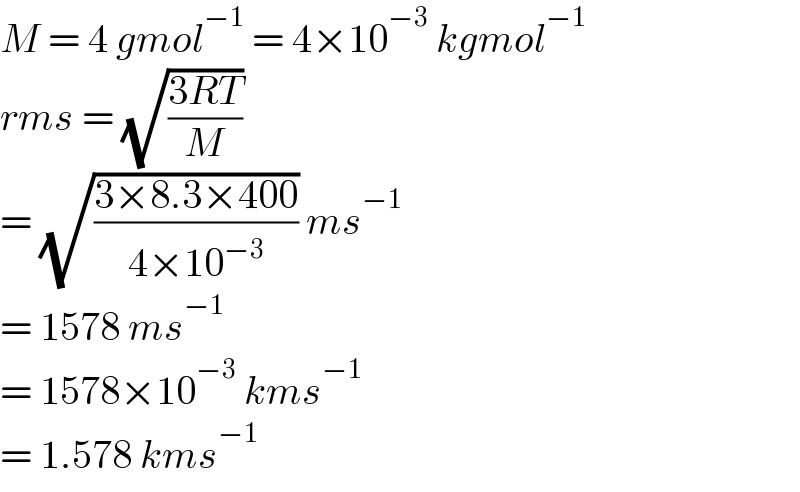 | ||
| ||
Commented by nadovic last updated on 11/Dec/22 | ||
 | ||